Chemists have a longstanding curiosity for heterocyclic amines, and ever since organic synthesis matured in the late nineteenth century, pyrrolidine rings have enticed researchers. The roots of 1-(Allyl)Pyrrolidine-2-Methylamine go back to classic alkylamine research. Large pharmaceutical labs developed analogs to test new leads in neurological therapies, especially during the medicinal chemistry renaissance of the late 1900s. Treatment options for central nervous system disorders often sprung from small tweaks to simple cyclic amines, and this molecule found its place in diverse screening libraries as methods for selective amination and functional group interconversions improved. Over the past couple of decades, investment in tailored cyclic amines only increased, so 1-(Allyl)Pyrrolidine-2-Methylamine naturally became more visible in both reference collections and process chemistry blueprints.
1-(Allyl)Pyrrolidine-2-Methylamine brings together a five-membered nitrogen ring and an allyl group with a methylamine function on the ring. Raw material supply for each precursor—allyl halides, pyrrolidine intermediates, methylamine reagents—flows from large-scale chemical production. Labs target this molecule for both its energized reactivity and manageable stability, striking a balance that allows easy use but stores well under proper conditions. Small-molecule researchers and process chemists draw on the options provided by this structure for both chemical and biological explorations. Its backbone remains approachable for further alteration, without presenting excessive synthetic headaches.
1-(Allyl)Pyrrolidine-2-Methylamine presents as a colorless to pale yellow liquid under ambient conditions. It offers low to moderate solubility in water, but dissolves well in standard organic solvents such as ether, dichloromethane, and ethanol. Boiling and melting points line up with related pyrrolidine frameworks, landing well above room temperature for melting, and just high enough that distillation applies for purification. The molecule tends to release a faint, amine-like odor, and batch quality connects to trace moisture levels. Acid/base behavior follows established trends for secondary amines, making it friendly for both acid extraction and salt formation when needed in process streams or intermediate isolation.
Bulk shipments arrive with certificates of analysis specifying assay (typically 97% or higher), main impurity profile, water content, and residue on ignition. Containers include GHS labels describing flammability, health hazards, and handling advice. MSDS documentation provides flashpoint and storage requirements. Some manufacturers offer NMR and GC/MS charts for batch verification; regulatory detail reflects national and regional transport and commerce laws. For projects in pharmaceutical or biotech spaces, regulatory-grade lots require audit trails for raw materials and cleaning logs for vessels.
Most routes start from pyrrolidine or substituted pyrrolidines. Alkylation proceeds with allyl bromide or chloride, often using base (like K2CO3) in polar, aprotic solvents. The methylamine group attaches either before or after allylation, by direct amination under pressure with methylamine gas, or via amide reduction when less reactive intermediates make more sense. After reaction completion, organic extraction removes impurities, and distillation or chromatography yields a clean product. Researchers accustomed to fine-tuning reaction schedules adjust pressure, temperature, and substrate ratios to favor desired ring and side chain selectivity, using TLC or LC/MS to monitor progress.
The nitrogen atoms in 1-(Allyl)Pyrrolidine-2-Methylamine allow significant flexibility for chemical manipulation. Acylation, sulfonation, or protection of the amine can generate derivatives ready for coupling or polymerization. The allyl group withstands moderate acids and bases but transforms under conditions promoting electrophilic addition, such as catalytic hydrogenation or epoxidation. Scientists seeking novel pharmaceutical scaffolds add functional groups via selective oxidation of the allyl chain or use cross-coupling with transition metal catalysts. The ring structure resists hydrolysis, but N-alkylation with longer chains or aryl halides introduces further diversity, facilitating structure-activity relationship (SAR) experiments in drug discovery.
Complex molecules accrue a handful of synonyms to aid their tracking and referencing. In catalogs, 1-(Allyl)Pyrrolidine-2-Methylamine appears as N-Allyl-2-methylaminopyrrolidine or 2-(Methylamino)-1-allylpyrrolidine. Internal project codes in pharmaceutical pipelines may obscure its structure. The chemical’s registry numbers (CAS, EC) help cross-search suppliers and regulatory reports. Some reference works and safety charts surface alternative spellings based on IUPAC translations or regional language conventions.
Chemists handling small alkylamines encounter skin and respiratory irritation quickly if protection lapses. 1-(Allyl)Pyrrolidine-2-Methylamine reacts sharply with oxidizers or acids, so separating it from peroxides or acidic waste cuts down on unwanted byproducts. Fume hoods become non-negotiable even in low-volume settings. Some batches emit eyes-tingling fumes, so gloves and goggles accompany every handling session. In scale-up, vapor suppression and air monitoring keep workplace exposure below threshold limits found in occupational guidelines. Storage uses sealed amber glass or lined steel drums, away from direct sunlight and moisture. Facilities pursue documented accident response plans incorporating eye wash stations, spill control, and neutralizing agents tailored to both flammable liquid and corrosive hazard classes.
Medicinal chemistry teams prize 1-(Allyl)Pyrrolidine-2-Methylamine for neuroactive compound research and as a base for synthetic tuning in structure-based drug design. Biotech labs look for its scaffold in screening programs exploring ion channel modulation or enzyme inhibition. Its derivative pathways plug directly into routes building up antivirals, CNS drugs, or agricultural actives. In material science, this amine’s backbone fits well for testing charge transport or polymer stabilization in specialty coatings. Analytical chemists reference it as a model system for method validation, tracking reactivity under stress, or benchmarking NMR/IR parameters. Some university teams pick up the molecule as a core for site-specific labeling, bioisosteric swaps, or late-stage functionalization exercises in training advanced students.
Activity in academic and industrial pipelines around pyrrolidine-based amines remains brisk. Teams optimize protocols for cost and yield, experimenting with green chemistry choices such as water-based media or reusable catalysts. Results from SAR campaigns push modifications with more polar or branched amine subunits, hoping to boost biological target affinity without sacrificing solubility. Researchers regularly report on new routes employing flow chemistry and continuous manufacturing for higher repeatability. The molecule also gets plugged into computational chemistry exercises, supporting virtual screening or property prediction for lead generation in multi-target projects.
Animal testing and in vitro models underpin most toxicity assessments. Early screens look at acute exposure, documenting effects on respiration, nervous system, and skin. Studies of 1-(Allyl)Pyrrolidine-2-Methylamine found moderate toxicity at high doses, mainly tied to its amine groups interacting with neurotransmission pathways. Chronic exposure research follows, watching for liver or renal stress after repeated dosing. Labs note genotoxicity and mutagenicity through Ames tests and chromosome aberration studies, generally flagging doses above those used in screening applications. Human data stays limited, but reports from synthetic labs emphasize the importance of avoiding skin contamination and inhalation. Regulatory agencies review these findings before issuing workplace limits or environmental discharge stipulations.
1-(Allyl)Pyrrolidine-2-Methylamine looks set for greater importance in both drug discovery and process engineering. With automation and AI-driven synthesis readouts transforming lab workflows, molecules with adaptable reactivity like this one find more opportunities in combinatorial settings. Green chemistry initiatives find this scaffold attractive because the core reactions require fewer toxic catalysts and lend themselves to water-based or recyclable solvent systems. The push for CNS therapeutics and advanced agricultural actives ensures continued screening and modification. As materials science demands new building blocks for electronics and smart coatings, the pyrrolidine-allyl-methylamine structure promises pathways to functionalized monomers. Regulatory trends demanding lower emissions and safer workplaces mean safer-by-design practices will feed back into every stage of this compound’s use, shaping packaging, labeling, and risk management for years ahead.
Anyone working in a chemistry lab knows synthetic molecules are the backbone of progress. Every new medicine, plastic, or industrial compound has a story that begins with a small, intricate molecule. Among these, 1-(Allyl)Pyrrolidine-2-Methylamine catches the attention of researchers, especially in medicinal chemistry and material science.
This compound is not something you’re likely to see at the local pharmacy or find in common cleaning products. Its structure—a pyrrolidine ring holding both an allyl group and a methylamine—makes it versatile in labs. What makes it stand out is how it offers a platform for making other molecules more complex. Chemists often look to such compounds as key intermediates. The presence of both nitrogen-rich and reactive groups means you can modify it in multiple directions.
Drug development can feel like assembling a puzzle, where each piece needs to fit just right. With 1-(Allyl)Pyrrolidine-2-Methylamine, medicinal chemists have a useful tool for piecing together molecules meant to interact with living systems. Many pharmaceuticals feature five-membered nitrogen rings because the body’s own chemistry responds well to that shape. This compound offers a starting point for building new drugs that might tackle pain, inflammation, or neurological problems. It becomes a foundation for synthesizing potential treatments by adding or swapping out groups on the molecule. Some research papers show the value of pyrrolidine derivatives in creating enzyme blockers and ligands for neurotransmitter receptors.
Sometimes it isn’t about the final product but about creating new pathways in chemical synthesis. I’ve seen talented organic chemists use compounds like this one as a scaffold for “combinatorial libraries.” The idea is simple: make hundreds or thousands of related compounds and look for the one with standout properties. With its reactive spots, this molecule fits well in these reactions, allowing creativity and problem-solving to shine in the lab.
Beyond the world of medicine, scientists look at molecules with unique reactivity for jobs in material science. By tweaking the allyl group or altering the pyrrolidine, materials scientists can generate special polymers, coatings, or resins. These might end up in electronics or new manufacturing techniques. I remember my own university days, seeing small tweaks to these sorts of molecules alter the strength and behavior of final materials in surprising ways. It’s never just about the base chemical; it’s what you build from it that often makes headlines in scientific journals.
New chemicals can raise safety flags. With compounds used in pharmaceutical research, safety and ethical oversight play a big role. Most reputable suppliers follow rules that trace who buys these chemicals and keep an eye on misuse. Researchers often highlight the importance of transparent reporting and following international guidelines. That protects both people working in the labs and those who will one day use products derived from their work.
Each step—from benchwork to market-ready product—depends on how well chemists harness intermediates like 1-(Allyl)Pyrrolidine-2-Methylamine. Rigor in documentation, collaboration across fields, and always asking tough questions about both risk and benefit, give society the chance to turn promising molecules into real-world advances.
Stepping into a chemistry lab always brings a certain energy—the click of glassware, scents lingering in the air, the buzz of collaboration. But with every new compound on the bench, a basic reflex kicks in: check the safety. 1-(Allyl)Pyrrolidine-2-Methylamine, with its mouthful of a name, rolls across chemical catalogs and research updates regularly. Not much comes up on major toxicology reports or safety data sheets, so anyone handling it faces some real uncertainty.
So much of the lab experience involves basic habits—gloves never far, safety goggles always smug on the nose, and the occasional frustration of sticky gloves. The minimal public information about 1-(Allyl)Pyrrolidine-2-Methylamine leaves familiar steps as the smartest route. Many amine compounds with similar structures cause irritation if they meet skin, eyes, or get inhaled. Years around amines taught me just how sharp even small vapors can feel on the nose and cheeks.
A few facts help connect the dots. The pyrrolidine ring gives a backbone to many pharmaceutical starting materials. Adding an allyl side chain and a methylamine group tends to hike up reactivity and—sometimes unexpectedly—boost toxicity. For amines, quick absorption through skin can start problems, and vapors have left me nauseous in the past. Take 1-(Allyl)Pyrrolidine-2-Methylamine’s close chemical cousins—several have MSDS warnings for acute toxicity if swallowed or inhaled.
I trust evidence over guesses, but habits from years in the lab say respect new chemicals. The lack of hard data on this substance raises a red flag. When a compound’s hazard profile is basically a blank page, treating it like its riskier relatives usually means fewer mistakes.
Rolling out the right precautions feels boring, but safety should outlast even the toughest deadlines. Engineering controls always form the home base—a working fume hood and ventilated workspace protect lungs and keep volatile smells away. Nitrile gloves have saved my skin more than once, especially with unknown organics. Washing hands after handling takes about thirty seconds but burns itself into memory after enough slips.
Labels on bottles matter. Even if the data’s thin, clear labeling and careful recordkeeping guard against accidental transfers or surprise splashes. Sharing hazard information with colleagues has stopped more accidents in my own lab experience than any formal poster campaign.
Fast internet brings journals and regulatory updates to our fingertips. Still, the science community drags its feet on posting new toxicological reports for up-and-coming chemicals. Researchers, chemical suppliers, and universities can collectively keep each other safer by uploading case studies, incident reports, or even just basic hazard notes online if traditional databases come up short.
People new to 1-(Allyl)Pyrrolidine-2-Methylamine should stick to fundamental safety habits and lean on the lessons learned from chemical analogues. The missing data won’t protect anyone. Experience, caution, and community communication will always have better odds in keeping lab hands safe.
Years ago, in my early time in a university chemistry lab, I watched someone store an amine-based compound wrong. They shoved it into a cheap plastic container, left it by a sunny window, and walked out thinking that was that. A week later, the stuff had discolored and gave off a weird odor. That moment stuck with me. Cutting corners usually backfires, especially with chemicals carrying reactive functional groups like 1-(Allyl)Pyrrolidine-2-Methylamine.
Organic amines bring their own set of quirks. This one, with an allyl group and a methylated pyrrolidine ring, doesn't simply sit on a shelf waiting to be used someday. Exposure to sunlight or heat can trigger unwanted changes. Moisture sneaking into containers can turn a clear sample into a yellow sticky mess. Vapor pressure can also climb at higher temps, which pushes vapors out of loosely closed vials.
There’s a serious safety angle too. Amines, by their nature, can be irritating to skin, eyes, and lungs. Vapors building up in a hot room can harm people breathing nearby. Keeping this compound chilled and tightly sealed isn't just a compliance box to tick. It means the people handling it every day aren’t rolling the dice with their health. I’ve seen careless storage lead to dangerous vapors filling up shared rooms—one time was all it took for lab policies to get rewritten.
Storing 1-(Allyl)Pyrrolidine-2-Methylamine at low temperatures slows down most reactions. I always keep similar amines well below room temperature, usually in a fridge or—if things are really volatile—a dedicated chemical freezer. Oxygen is another enemy, especially if the sample stays open to air. Once, I cracked open an old bottle and found nothing but smell and sludge. Inert gas, like nitrogen or argon, can help keep the content fresh if opening the container often.
Don’t forget about moisture. Even with a screw cap, humidity can sneak in. Silica gel packets or other drying agents inside the outer storage box make a difference. On the rare moments I've managed inventory in a chemistry storeroom, the sealed vials with drying pouches always looked better, even after months. Desiccators—especially ones with visible color-changing indicators—give confidence the sample isn’t degrading quietly.
Chasing after convenience or saving steps rarely pays off in the long run. Lax storage can turn usable material into hazardous waste, costing more in disposal fees and cleanup than was saved by skipping the temperature log. Continuous monitoring—labeling vials with opening dates, tracking temperature with cheap digital loggers—shows patterns before anything gets lost. In my time working with regulatory audits, careful logs often earned a laboratory a pass during surprise inspections.
From a practical side, keeping chemicals right means consistent research and reliable results. Quality control falls apart fast if the raw materials keep changing texture or potency. A well-run operation stays ahead by keeping everything cold, sealed, and dry—and making it known that these rules are about protecting people, data, and the investment in the work itself.
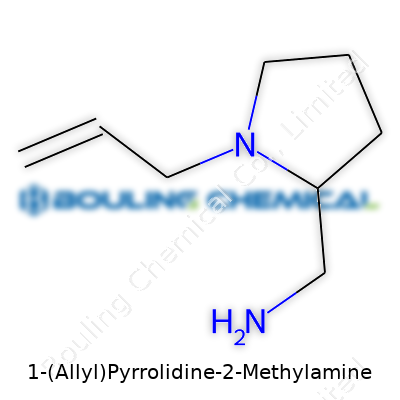
1-(Allyl)Pyrrolidine-2-methylamine has a name that paints a puzzle for many, but an experienced eye turns it into a blueprint. On one end stands pyrrolidine, a five-membered ring with four carbons and a nitrogen. Scientists call this ring a handy building block in pharmaceuticals. Attach a methylamine group at the second position of the ring, and then put an allyl group—a chain with three carbons and a little double bond—at the first position. The chemical shorthand might be C8H16N2.
Drawing it out, the pyrrolidine ring forms the backbone. The allyl group (–CH2–CH=CH2) connects to the nitrogen atom, bringing flexibility and increased reactivity. Methylamine (–CH2NH2) hooks onto the carbon directly next to the nitrogen, at position two. The arrangement influences how this molecule interacts with biological targets, including enzymes and receptors that shape body chemistry.
Chemists and drug developers see promise in these types of structures. Pyrrolidine rings show up in natural products and lab-derived drugs for neurological disorders. Add an allyl group, and reactivity changes, possibly helping the compound sneak past natural defenses or bind more snugly to its target. Methylamine groups have punch too, adding hydrogen bonding that sharpens interaction with enzymes.
Thinking back to lab days, visualizing these structures with models often brought challenges to life—rotating rings and dangling chains can mean the difference between a potential medicine and a failed experiment. The orientation matters, sometimes flipping a molecule’s activity from helpful to harmful.
Working with nitrogen-heavy molecules brings risks and rewards. Nitrogen atoms in rings often carry lone pairs of electrons that play a part in reactivity. Experienced chemists know that tweaking the chain length or swapping places of the side groups can change behavior completely. Making this compound requires careful planning, from choosing starting materials to running reactions in dry, oxygen-free environments. Allyl groups demand respect during synthesis—underestimating their reactivity can lead to accidents. Labs enforce proper gloves and fume hoods, highlighting safety alongside curiosity.
These molecular architectures appear in drug development pipelines. Some variations of pyrrolidine are found in FDA-approved treatments, while others ride the fringes due to safety questions or legal issues. Chemists recognize the potential for misuse, so strict regulations often surround their handling. Transparency in research reporting and following established safety procedures help preserve public trust.
Training emerging scientists to recognize both the opportunity and responsibility in working with such molecules shapes the future of the field. Raw curiosity pushes discovery, but ethical concerns and patient safety remain front and center. Sharing accurate chemical information and encouraging responsible stewardship ensures that discoveries benefit society rather than threaten it.
Before anyone even thinks about placing an order for something with a name like 1-(Allyl)Pyrrolidine-2-Methylamine, some big questions pop up. First one: is someone getting into legal trouble trying to buy it? This isn’t as straightforward as picking up cold medicine at the local pharmacy. In some circles, obscure molecules like this wind up on lists that nobody wants to be on—think controlled substances, drug precursor regulations, or flagged import/export criteria.
Authorities care about chemicals that could turn into something illegal. That’s not paranoia; it’s real policy. If a substance looks like it might fit into a recipe for dangerous drugs, regulators flag it. Sometimes, that means an outright ban. Other times, they want extensive documentation before anything ships. The world doesn’t follow a single rulebook, either. In the United States, federal agencies and some state law enforcement treat this stuff carefully. Europe features similar hurdles, often tapping into the same UN conventions that shaped drug law elsewhere. Australia, Japan, and China keep close eyes on novel chemical imports, too.
It took getting involved in a chemistry lab to see these rules up close. Once, I watched our material orders delayed for weeks. Turns out, an obscure molecule rolled down a regulatory checklist somewhere. Distributors wanted records, permits, background explanations. Why does a small suburban lab need this? The answer had to be sharp. And even then, sometimes the answer was no.
Legal lines aren’t the only obstacle. Reputable chemical suppliers don’t want to risk their business. They study demand. They check company credentials. They verify intended use, especially with unfamiliar customers or chemicals that raise eyebrows. Reputable vendors ask these questions to keep things legitimate. Even without a specific ban, access narrows.
Many mail-order sites claim global reach, but they don’t deliver everything, everywhere. Sometimes, a click-bait order page lets you start to buy almost anything. At checkout, terms appear. Suddenly, extra documentation pops up, or the order quietly gets cancelled. The practical barrier here outpaces the legal one in many ways, especially for buyers without credentials or a professional reason.
It’s not dramatic to say that public safety sits in the balance. Chemicals like this aren’t sold for making dinner. People abuse gaps in the law to dodge restrictions, tweaking a molecule here and there. Governments then race to rewrite the rules. This cycle happens with everything from synthetic cannabinoids to designer amphetamines.
Lawmakers can experiment with “analogue” laws—regulation that covers not just named substances, but anything chemically similar enough to cause the same risks. That keeps smart chemistry from outpacing the law. On the other side, researchers worry about losing access to chemicals that help medicine, diagnostics, or industry. The challenge: protect the public without blocking real science.
Safety means more than following the law. It means tracking sourcing, knowing a supplier’s reputation, understanding storage rules, and dealing with disposal carefully. Professional environments use chemical safety officers for a reason. I’ve watched teams pour over each step for something as simple as ethanol, let alone an obscure amine. If someone cares about safety and legality, finding out the real story behind that chemical order means more than ticking a box. It means learning the unwritten rules, knowing the reputation of every player, and being honest about the consequences. If the answers aren’t clear, the best move is to ask—and keep asking—until every legal and ethical angle gets checked.
| Names | |
| Preferred IUPAC name | N-Prop-2-enylpyrrolidine-2-methanamine |
| Other names |
1-Allyl-2-(methylamino)pyrrolidine N-Methyl-1-allylpyrrolidin-2-amine |
| Pronunciation | /ˈwʌn ˈæl.ɪl paɪˈrɒl.ɪˌdiːn tuː ˈmɛθ.ɪl.əˌmiːn/ |
| Identifiers | |
| CAS Number | 1350761-12-6 |
| 3D model (JSmol) | `C1CC(NC)CC1C=CC` |
| Beilstein Reference | 10259022 |
| ChEBI | CHEBI:189476 |
| ChEMBL | CHEMBL3726194 |
| ChemSpider | 29178620 |
| DrugBank | DB08378 |
| ECHA InfoCard | 08e233c8-fcda-42af-a3b3-0e8fe7a5c1e7 |
| Gmelin Reference | 1676767 |
| KEGG | C19561 |
| MeSH | 1-(Allyl)Pyrrolidine-2-Methylamine" does not have a specific MeSH (Medical Subject Headings) term assigned. |
| PubChem CID | 102278422 |
| RTECS number | UD6509000 |
| UNII | W4Y10442GW |
| UN number | UN No. : "UN1993 |
| Properties | |
| Chemical formula | C8H16N2 |
| Molar mass | 126.22 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.928 g/cm3 |
| log P | 0.9 |
| Vapor pressure | 0.126 mmHg at 25°C |
| Acidity (pKa) | pKa ≈ 10.7 |
| Basicity (pKb) | 3.22 |
| Refractive index (nD) | 1.480 |
| Dipole moment | 2.35 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | N06BX13 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 86.5 °C |
| NIOSH | RN 171674-97-4 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| Related compounds | |
| Related compounds |
Pyrrolidine 2-Methylpyrrolidine 1-Allylpyrrolidine N-Methylpyrrolidine Pyrrolidine-2-carboxamide N-Allylmethylamine 1-(Allyl)piperidine |