Long before high-throughput screening sped up drug discovery, chemists relied on methodical trial-and-error work. 1-Acetyl-4-(4-Hydroxyphenyl)Piperazine reflects the spirit of these early scientific endeavors. The compound’s backstory reaches into the roots of medicinal chemistry, when researchers experimented with piperazine derivatives to harness their central nervous system effects and tap their potential as intermediates for active pharmaceuticals. Interest in piperazine scaffolds grew in the late 1900s, as their flexibility and reactivity allowed medicinal chemists to construct diverse bioactive molecules. Its structure, marked by an acetyl group on the nitrogen and a hydroxyphenyl moiety, became a foundation in drug structure-activity relationship studies. This opened doors for exploring modifications that altered physiochemical properties and bioactivity.
1-Acetyl-4-(4-Hydroxyphenyl)Piperazine often appears as a white or off-white powder. It strikes a balance between water solubility and lipophilicity, which has fueled its use as a synthetic intermediate in research. Manufacturers distribute it for research, formulation development, and as a test substrate in analytical method validation. Its accessible functionality on both the piperazine ring and the aromatic system makes it a handy piece for academic and industrial labs. Its multiples points of reactivity offer chemists avenues for novel drug design as well as materials science work.
With a molecular formula of C12H16N2O2, 1-Acetyl-4-(4-Hydroxyphenyl)Piperazine presents a moderate molecular weight of about 220 grams per mole. The solid melts in the range of 109-112°C. In water, it displays low to moderate solubility, and it tends to dissolve much more readily in polar aprotic solvents such as DMSO. The compound features a neutral or slightly acidic phenolic group, which affects both its solubility and its behavior in organic reactions. Ultraviolet light detects this compound efficiently because the aromatic ring absorbs in the relevant regions. In routine storage under cool, dry conditions, the powder remains stable for extended periods, with little tendency toward degradation.
Producers offer this compound at a reported purity exceeding 98%. Analytical labs certify each lot through several techniques, including proton NMR and HPLC. Some distributors follow industry standards for container labeling, using globally harmonized system icons and hazard rankings. The labels highlight possible irritation and recommend basic precautions for handling, such as wearing gloves and eye protection. Trace impurities, if noted at all, fall below 1% and rarely impact research outcomes.
Synthesis commonly starts from 4-hydroxyaniline, which undergoes alkylation with a piperazine derivative, often via a nucleophilic substitution reaction. An acetylation step usually follows, using acetic anhydride under mild conditions and in the presence of a base such as sodium acetate or pyridine. The resulting product is purified by recrystallization from water-ethanol mixtures. Operators may rely on vacuum drying to ensure removal of solvents, leaving a dry, stable product for downstream use. Some laboratories choose alternative one-pot approaches to simplify and streamline the process, anchoring on operational simplicity and avoiding hazardous reagents.
This compound's core offers chemists a set of reliable modification sites. The phenolic group undergoes etherification and esterification, often to tune hydrophobicity or to attach targeting moieties for biological assays. The acetyl functionality can be removed or exchanged for other acyl groups, providing routes to structurally diverse analogues without altering the core piperazine system. Electrophilic aromatic substitution on the phenyl ring, such as halogenation or nitration, brings further diversity for structure-activity studies. The piperazine ring itself supports N-dealkylation or N-oxidation, giving rise to metabolites that researchers track in drug metabolism studies. These synthetic handles make the molecule attractive for both single compound synthesis and as a feedstock for combinatorial libraries.
Across the chemical and pharmaceutical industries, this molecule might travel under several names. Some catalogs list it as N-Acetyl-4-(4-Hydroxyphenyl)piperazine. The shorthand abbreviation 4-HO-APP is common in some research communities. Chemical supply houses occasionally use the systematic name 1-Acetyl-4-(p-hydroxyphenyl)piperazine in line with IUPAC conventions. Other synonyms, like N-[4-(4-Hydroxyphenyl)piperazin-1-yl]acetamide, may appear in patent or regulatory filings. Keeping track of these synonyms helps navigate the maze of literature and purchase orders.
Working with nitrogen-containing aromatics always deserves respect for safety protocols. This compound, though less hazardous than some aromatic amines, can still introduce risks. Inhalation or skin contact might trigger irritation in sensitive individuals. Good laboratory protocols call for use of gloves, protective eyewear, and adequate ventilation, especially if handling gram-scale quantities. Any accidental release onto work surfaces or skin should prompt prompt washing with soap and water. Waste material—whether solid residue or rinse—should go into chemical waste streams, not general trash or drains. Most labs find that careful adherence to written SOPs and regular safety reviews prevent accidents, even with novel compounds.
Researchers often turn to 1-Acetyl-4-(4-Hydroxyphenyl)Piperazine as a versatile intermediate. Its piperazine motif attracts attention in drug discovery, since many CNS-active agents share this backbone. Scientists use it as a template when designing serotonin receptor ligands or when probing the binding characteristics of enzyme inhibitors. Analysts in chemical testing labs also depend on it as a test probe to calibrate instruments for complex drug sample work. In materials research, the phenol group’s reactivity opens up possibilities for linking to polymers or surfaces, an application relevant in sensor development and analytical chemistry.
The R&D community has watched this compound with keen interest, driven by the realization that piperazine derivatives act as privileged structures in medicinal chemistry. Lab teams employ it as a chemical handle when building libraries for screening campaigns, targeting conditions ranging from psychiatric disorders to metabolic disease. Structural modifications around the hydroxyphenyl segment inspire new approaches to selectivity and metabolic stability. Published studies, especially in the past decade, reflect a surge in structural analogues aimed at hitting newer targets, including receptor subtypes and protein interaction interfaces. Each new variant helps pin down which atomic tweaks drive therapeutic outcomes or liabilities, so the compound’s popularity rarely wanes.
Toxicological screenings shed light on the limits and potential of molecules like 1-Acetyl-4-(4-Hydroxyphenyl)Piperazine. Preclinical data, often published as part of broader piperazine class exploration, point to mild to moderate toxicity at higher exposures in cell and animal models. Investigators flag the potential for liver enzyme interactions, a hallmark issue with aromatic amines and piperazine ring structures. The phenolic group, though generally seen as benign, can bioactivate in some metabolic contexts, which leads scientists to track oxidative byproducts closely. Regulatory bodies advise focusing on dose ranges, exposure frequencies, and possible cumulative effects—steps that mirror standard industry practice. The body of evidence gathered so far supports the view that cautious, well-controlled lab practice keeps risks well managed.
Continued advances in molecular biology, drug design, and analytical chemistry keep driving up the profile of specialty intermediates like 1-Acetyl-4-(4-Hydroxyphenyl)Piperazine. With every fresh insight into receptor function or enzyme selectivity, the need for sturdy, modifiable scaffolds grows. Many startups and university labs have begun exploring these modified piperazines not only as drug leads, but also as biosensor elements and chemical tags. Software-driven molecule screening and AI-predicted binding dynamics could soon unlock new roles for these compounds in both healthcare and high-tech manufacturing. As investment pours in to back early-stage research, piperazine-based intermediates stand poised to deliver innovation faster and help address everything from therapeutic gaps to diagnostics challenges.
If you spend time around chemists or pharmacologists, you will hear names like 1-Acetyl-4-(4-Hydroxyphenyl)piperazine. The name is a mouthful but it matters in human health. This compound attracts attention because it forms the backbone for a number of potential medications. Researchers use it when they need a solid scaffold for drug design, especially in developing treatments for neuropsychiatric and pain-related disorders.
Modern medicine takes small pieces, like piperazine rings, and mixes them with other components to create new pharmaceuticals. 1-Acetyl-4-(4-Hydroxyphenyl)piperazine acts as one of these useful intermediates. Its structure — a piperazine ring with both an acetyl group and a hydroxyphenyl moiety — brings together chemical properties that can interact with various biological targets.
Several research papers highlight this compound for synthesizing selective serotonin receptor ligands. SSRIs, the medications that help millions cope with depression and anxiety, rely on related scaffolds to bind more strongly to certain receptors in the brain. Compounds featuring the hydroxyphenyl group often show strong affinity for these targets. In simpler terms, chemists see this synthetic piece as a shortcut to designing molecules that fit into the brain’s lock-and-key system for mood and pain control.
Pain management and psychiatry continue to search for medications with fewer side effects. The traditional drugs sometimes leave people feeling sedated or addicted. Scientists working on alternatives often try out new chemical blueprints—including altered versions of 1-Acetyl-4-(4-Hydroxyphenyl)piperazine—to reach the right balance between effectiveness and safety.
Some published data reveals how related compounds activate or block pain pathways, or tweak serotonin signals. The hydroxyphenyl group encourages interaction with serotonin 5-HT1A and 5-HT2A receptors. Evidence shows that even a simple acetyl group can change how a molecule behaves in the body: it affects solubility, absorption, and the way the liver processes medication. This level of fine-tuning helps researchers get closer to drugs that improve lives without unwanted baggage.
The raw chemical market booms in China, where suppliers produce intermediates like this piperazine for labs around the world. Access to affordable synthetic building blocks means more small companies and university labs can tackle tough health issues, from pain management to mental health. The spread of such intermediates also raises questions about regulation. As these chemicals serve as the foundation for controlled medications, proper oversight helps cut down on misuse or illegal production.
With any active molecular fragment, there’s room for caution. Powerful building blocks can sometimes end up in the wrong hands, contributing to illegal drug synthesis. Doctors, chemists, and policymakers need close collaboration to keep scientific progress focused on treating disease. Stricter tracking of sales and usage, combined with responsible disclosure from suppliers, can keep innovation flowing while guarding against negative consequences.
Seeing what talented researchers and companies do with 1-Acetyl-4-(4-Hydroxyphenyl)piperazine in the coming years will tell us a lot about the future of brain and pain medicine. Advances in chemistry always bring new hope and new questions.
Anyone who’s spent time in a lab or on an industrial floor knows every compound brings its own hazards. Even compounds that look harmless can turn nasty fast. I’ve seen colleagues rush, skip goggles, and end up with chemical burns. No job or experiment runs smoothly if someone winds up in the hospital. Knowing a compound’s risks should guide every move. Even if the material is new, a quick check of its chemical data sheet pays off. There’s usually a section on fire, skin contact, and inhalation dangers. I recommend keeping that sheet handy, either printed or bookmarked.
Personal protective equipment takes a minute to put on but can save skin, eyes, and lungs from serious harm. Basic PPE includes goggles, gloves, and a lab coat. Certain compounds demand more: double gloves, splash-resistant aprons, or even a full-face shield. If something creates fine dust or vapors, a respirator or working in a fume hood matters a lot. I remember cleaning up a spill once, thinking a mask would slow me down. Hours later, I coughed so hard I couldn’t finish the shift. Since then, I never skip the mask.
Shutting container lids and labeling everything properly sounds simple, but it’s where mistakes show up. People forget where they set things down, or someone else walks in, not realizing what’s inside a beaker. I’ve seen spills happen just because a lid wasn’t tight. After every use, I make a habit of checking lids, labeling containers with dates and warnings, and never mixing old waste with unknown liquids. Labs and workshops get busy, but neat storage and clear labels stop confusion before it starts.
Spills can happen in the best labs. Having a spill kit nearby—one with absorbent pads, neutralizers, and gloves—makes any emergency less scary. I suggest everyone run through a quick drill every couple of months. Know where to find eyewash stations, emergency showers, and exits. If something spills on your skin, rinse with water right away, then get medical evaluation. It’s easy to dismiss a splash as nothing, but some compounds burn or cause reactions after a few minutes.
Strong-smelling or volatile compounds can sneak up on you. Opening a window might not cut it. I always push for using a fume hood, especially if there’s vapor involved. Fume hoods keep dangerous fumes away from your nose and eyes. Some folks try shortcuts, but ventilation gear exists for good reason. Chronic exposure to some chemicals increases the risk of lung damage or worse. Proper air flow isn’t just comfort—it’s health protection.
It helps to tap into a network of experienced colleagues when handling new compounds. If a team member has used a certain reagent for years, their tips can be as valuable as the manufacturer’s guidelines. Peer experience often reveals shortcut mistakes that safety manuals miss. Listening and sharing close calls helps everyone stay safer. In my work, that community mindset saves time, mistakes, and—sometimes—lives.
In the lab, purity isn’t some fancy label reserved for scientists who love details. It tells me how trustworthy a product really is—if I’m holding a bottle that says 99.9%, I want to know there’s hardly anything hiding in that other 0.1%. Purity decides whether my results are real or skewed. If a chemical slips in with some mystery impurity, my whole test could head off in a direction nobody expects. Even a few stray molecules can ruin a pharmaceutical batch or make food unsafe. That’s not an exaggeration—I’ve watched people sift through stacks of certificates, all to find the tiniest unwanted byproduct before a medicine even leaves the factory.
Companies claiming their product is “pure” don’t just trust the supplier’s word. They break out high-precision tools. Chromatography separates the good from the bad. Spectroscopy spots chemical footprints down to the parts-per-million. Sometimes mass spectrometry jumps in and looks for even tinier traces. These tests leave very little room for “guessing.” I once saw a supplier drop off a drum for quality testing, only for our team to spot a trace of another compound using just a well-timed gas chromatography analysis. They pulled the whole shipment off the line. That’s how important single digits can become.
Packing lines at food factories watch for purity as if lives depend on it—because they do. Every product with an ingredient list should offer more than marketing promises. A company making vitamins or drink powders checks for heavy metals, pesticides, and those sneaky fillers. If they find anything out of place, the entire batch turns to waste. Consumers trust every ingredient has cleared this hurdle long before a package lands on the shelf. For me, reading the certificate of analysis feels a bit like looking under the hood of a car before driving away—sometimes the gloss on the outside hides a crack waiting to form.
No factory churns out perfect batches every time. Machines drift off calibration. Staff get tired. Raw materials ship in at lower grades during supply crunches. That’s the real-world stuff I’ve seen—where pressure for speed dents the focus on quality checks. Purity slips can mean recalls or even hospital visits if contamination gets past every checkpoint. After big events, some companies add redundant testing or switch suppliers altogether. It’s a constant battle between cost, speed, and the safety line nobody wants to cross.
Every time someone asks about purity, they deserve straight answers, not fuzzy guarantees. The best suppliers hand over up-to-date certificates, batch numbers, and proof of each test. Sometimes it’s on a secure website, sometimes packed with the product. Labs can do spot-checks for new suppliers. People in the field, like farmers or small brand owners, stretch their resources further by working with third-party testing labs. Regulators crack down on shortcuts, making it easier for everyone to check certifications and complaint histories online.
Pushing for higher purity isn’t just about numbers or perfection—it’s a health issue, a business saver, and a trust builder. I’ve seen what happens when even small steps are skipped, and the fallout isn’t worth the risk. Getting those percentages right keeps everyone, from the factory team to the neighborhood shopper, a little safer each day.
Years spent working in research labs taught me that it takes more than just a tidy shelf to keep chemicals out of trouble. Some compounds break down under sunlight, others react with the air, and plenty do not like neighbors. 1-Acetyl-4-(4-Hydroxyphenyl)Piperazine falls in the category of substances where extra care makes a real difference. Its structure signals sensitivity, especially where that exposed hydroxy group sticks out. That group can attract moisture from the air and start mild chemical changes before anyone notices.
The first routine I always followed: store such compounds in a cool, dry place. A drawer, cupboard, or even a fridge, so long as temperature remains steady and low. Many labs use dedicated refrigerators at 2–8°C, reserving them for temperature-sensitive chemicals. Regular fridges risk contamination from food or accidental temperature spikes. I always placed a clear label—compound name and date—instead of relying on memory. Humidity ruins many reagents, and excess water tends to creep in during careless storage, so putting an adequate desiccant inside the storage container adds another layer of protection.
One thing that’s easy to overlook is the effect of light. Certain chemicals, especially those with aromatic rings or exposed hydroxy groups, break down when exposed to sunlight or even strong overhead bulbs. I recall a colleague who lost an entire batch after leaving a vial near a sunny window. Amber glass bottles or light-blocking wrappers make storage safer—they prevent the slow, silent breakdown that can ruin months of work.
Oxygen in the air plays tricks too. Opening a bottle and leaving it sitting out can start a slow reaction, sometimes forming peroxides or byproducts harmful to both the substance and anyone working with it. I learned to open containers only long enough to weigh out what I needed, then seal it tightly—often using parafilm, which holds back moisture and air much better than regular caps alone.
No matter how fancy the equipment, human mistakes account for many accidents and ruined chemicals. I trained myself and younger lab members to wear gloves, avoid cross-contamination, and never guess measurements. One afternoon, I watched someone accidentally drip water into an open vial of a moisture-sensitive compound—ruined in seconds. Personal stories like that drive home the point: always keep containers closed when not in use, label everything, and segregate storage by risk—not by how convenient it seems on the shelf.
Proper packaging keeps things safe. Polyethylene bottles work for stable powders, but anything with a known reactivity or sensitivity goes in glass or high-barrier plastic. Seals matter—a crimped aluminum cap or thick screw-top beats thin plastic every time. Some suppliers even double-bag or add an inert gas layer. That’s not excessive; oxygen and water travel through plastic slowly, so extra barriers pay off if you won’t use the substance quickly.
Relying on low humidity and stable temperatures works, but in hot or damp climates, standard storage might not cut it. Dry cabinets with humidity controls or portable desiccators help. If power outages happen often, chemical storage in insulated boxes with desiccants or using silica packs adds a backup. Good practice also means checking old stocks: if a vial shows clumping, discoloration, or unexpected smells, best to replace it rather than risk wrecking an experiment or endangering staff.
Safe storage turns chemistry from a danger into a daily routine. I respect chemicals not as potential hazards, but as tools for discovery—so long as I match their quirks with practical habits and solid equipment. Experience shows: cuts in safety or storage rarely save time or money in the long run. In my world, careful storage isn't an optional step; it's the backbone of sound research and workplace safety.
Anyone building a business around chemistry knows supply isn’t just about technical details. The regular grind involves asking blunt questions. So, is this compound available in bulk, or is the project over before it starts? This isn’t just a procurement issue—what happens in the supply chain can shape careers, business prospects, and research timelines. Labs waste far too many hours chasing sources just to sign off a single order.
The wider world doesn’t pay much attention to the dizzying dance behind a simple drum of chemicals. But try bringing a new drug into clinical trials, or keeping a water treatment plant running during a pandemic. Suddenly, the question of who stocks something at a hundred kilograms per shipment keeps you up at night. Many producers list compounds on paper but limit batches due to production bottlenecks, raw material prices, or licensing headaches. A supplier can say “in stock” but mean fifty grams on a shelf, not actual barrels.
Every business with skin in this game checks the pedigree on bulk suppliers. Shady resellers or middlemen can hold back legitimate scale. Lean times reveal that reliable bulk availability means understanding upstream sourcing, freight logistics, and the regulatory checkpoints stuck between port and plant. Years ago, watching a colleague’s pilot project grind to a halt over a simple shortage of ammonium sulfate drove that home hard. Nothing galls like paying project teams while powder sits on a ship outside a clogged port.
Every order forms one more link in a value chain built around trust. The smart buyers go beyond websites and get on the phone. They want to know if a chemical comes from a real facility, or a “virtual” distributor farming out orders. Scrappy procurement pros ask about batch size, purity, global warehousing, and delivery records. I always look for suppliers who post certificates of analysis right up front, and stick with partners who send honest answers—good or bad—about what’s possible this fiscal quarter.
Bulk ordering doesn’t simply come down to price. Reality kicks in by the truckload as soon as hazardous goods, export controls, or labeling requirements enter the mix. Quality lapses on batch consistency can cost whole months’ worth of testing. I’ve seen promising projects get shelved because finance forced a low-cost purchase, only for the batch to flunk re-certification. The hidden cost of cheap powder often looks like missed production slots and churned inventory, not just line items on a spreadsheet.
Anyone facing scale-up should start by building direct lines to proven suppliers. Think about third-party audits, site visits, or insured shipments. Public reports of recalls or supply interruption signal risk—solid data must come from somewhere, and it never hurts to cross-check with industry forums or buyers in similar sectors. By maintaining open dialogue and demanding real transparency, I’ve sidestepped disaster more than once. If supply seems too easy, dig deep; real bulk deals come with paperwork, customization, and clear commitments from both sides. Consistency, accountability, and open records tell you more than glossy catalogs ever do.
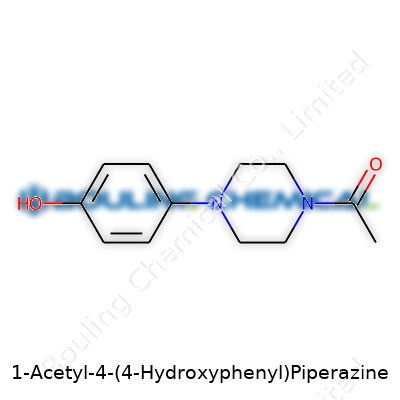
| Names | |
| Preferred IUPAC name | 1-[4-(4-Hydroxyphenyl)piperazin-1-yl]ethan-1-one |
| Other names |
1-Acetyl-4-(4-hydroxyphenyl)piperazine 1-(4-Acetylpiperazin-1-yl)phenol 4-(4-Hydroxyphenyl)-1-acetylpiperazine |
| Pronunciation | /waɪˈæs.ɪˌtiːl.fɔːr.fɔːr.haɪˈdrɒk.siˌfiː.nəl.paɪˈpɛr.əˌziːn/ |
| Identifiers | |
| CAS Number | [67859-67-6] |
| 3D model (JSmol) | `3D model (JSmol)` string for **1-Acetyl-4-(4-Hydroxyphenyl)Piperazine**: ``` CC(=O)N1CCN(CC1)C2=CC=C(C=C2)O ``` |
| Beilstein Reference | 1310703 |
| ChEBI | CHEBI:77469 |
| ChEMBL | CHEMBL2109286 |
| ChemSpider | 21561168 |
| DrugBank | DB08215 |
| ECHA InfoCard | ECHA InfoCard: 100_110_672 |
| EC Number | 62025-16-3 |
| Gmelin Reference | 100554 |
| KEGG | C11772 |
| MeSH | D017372 |
| PubChem CID | 131820 |
| RTECS number | GV9485000 |
| UNII | 71Z88A1MI6 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C12H16N2O2 |
| Molar mass | 250.30 g/mol |
| Appearance | White to off-white solid |
| Odor | odorless |
| Density | 1.16 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 1.02 |
| Vapor pressure | 4.11E-7 mmHg at 25°C |
| Acidity (pKa) | 9.76 |
| Basicity (pKb) | pKb = 5.98 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.624 |
| Dipole moment | 4.93 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -480.6 kJ/mol |
| Pharmacology | |
| ATC code | N06AX11 |
| Hazards | |
| Main hazards | Causes skin, eye, and respiratory irritation. Harmful if swallowed. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| Flash point | > 247.0 °C |
| LD50 (median dose) | LD50 (median dose) Oral - rat - 2,000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g,25g,100g |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Paracetamol Phenacetin 4-Hydroxyacetanilide 4-Phenylpiperazine 1-Acetylpiperazine 4-(4-Hydroxyphenyl)piperidine |