People have worked with nitrogen-containing rings for over a century, so it’s not surprising that 1,4-dimethylpiperazine appeared early in the story of modern organic chemistry. In the mid-20th century, labs began exploring the piperazine scaffold. They sought out new drugs and synthetic pathways, but they also chased ways to tune these molecules for various industrial and research applications. Chemists soon discovered that replacing the hydrogen atoms on nitrogen with methyl groups shaped the properties and expanded the molecule’s uses beyond what the original piperazine could offer. Over the years, this compound has been tucked away in patent disclosures, lab notebooks, and the glassware of drug-discovery programs. Every generation of researchers seems to rediscover how versatile it can be.
1,4-Dimethylpiperazine brings together a six-membered saturated ring and methyl groups on each nitrogen. It falls under the simple heterocycles family. Labs order it as a base, intermediate, or reagent. Specialty chemical makers produce it by the drum for process chemistry, so you see it on the shelf of chemical suppliers in a range of purities. Its reach extends from basic research to pilot plant and commercial scale. The product has never attracted hype, but its reliability and adaptability in synthesis keep it in steady circulation.
At room temperature, 1,4-dimethylpiperazine forms a colorless liquid or low-melting solid, depending on storage and purity. The smell is faint and amine-like, unmistakable in an organic lab. The compound boils around 136 °C under atmospheric pressure. It dissolves well in many organic solvents like ethanol, ether, and chloroform. Water solubility decreases compared to plain piperazine, due to those extra methyl groups making it less polar. Its structure, thanks to methylation at both nitrogens, shields it from reactions like protonation, so it stays free-base under normal conditions. This makes a difference in both handling and downstream chemistry.
Chemical suppliers generally deliver 1,4-dimethylpiperazine with purity listed above 98%. Labels show its CAS number (106-58-1), molecular formula (C6H14N2), and structure as a six-membered ring with N-methyl groups facing opposite directions. Alongside handling guidelines, you’ll see hazard statements reflecting its classification as a flammable liquid with acute toxicity risks on exposure. Storage suggestions often highlight a tight-sealing cap and protection from heat, sparks, and incompatible chemicals like strong oxidizers. Shipment complies with international hazardous materials codes and includes clear documentation for traceability.
Industry often prepares 1,4-dimethylpiperazine by methylating piperazine itself. Common methods use methyl halides such as methyl iodide or methyl bromide under basic conditions. Sometimes, more environmentally friendly methylating agents like dimethyl sulfate or dimethyl carbonate get the nod for greener chemistry initiatives. The methylation runs in polar solvents such as acetonitrile or DMF and needs a strong base to catch the acid byproduct. Once the reaction wraps up, chemists extract and distill the product, taking care to separate it from over-methylated or partially reacted material.
Methyl groups on each nitrogen block many side reactions, so 1,4-dimethylpiperazine acts as a stable base or nucleophile in organic synthesis. It resists acidic and basic hydrolysis. Still, the ring stays open to transformations at the carbon atoms, such as oxidation or alkylation under strong conditions. Some researchers have used it as a precursor to more complex heterocycles, or as a building block for fine chemicals and active pharmaceutical ingredients (APIs). Reaction energies vary with context, but most labs appreciate this compound for the way it offers predictable behavior without unexpected side products.
You’ll find 1,4-dimethylpiperazine under several names. Some catalogues call it N,N'-dimethylpiperazine. Others abbreviate it as DMPZ. International suppliers sometimes list it as Piperazine, 1,4-dimethyl derivative. Regulatory filings stick with its IUPAC name or CAS number, while R&D teams know it by shorthand in project documents. Whatever the moniker, the structure remains the same and the paperwork connects back to the same molecular identity. This helps buyers and lab techs check compatibility for process documentation or cross-border supply chain compliance.
Direct contact with 1,4-dimethylpiperazine can irritate skin, eyes, and respiratory system. Labs and facilities require gloves, goggles, and good ventilation. Spills on skin need prompt washing with soap and water. Inhalation calls for medical attention if symptoms occur. As a flammable liquid, it stays away from ignition points, sparks, and open flames. Waste goes into closed chemical containers and follows hazardous waste protocols. Exposure monitoring and workplace training maintain a healthy margin of safety for users, guided by national and international occupational standards.
As far as uses go, 1,4-dimethylpiperazine covers quite a bit of territory. Medicinal chemistry labs use it to create small molecule libraries and build up drug candidates. Its chemical stability and unique geometry make it useful for designing molecules targeting neurotransmitter systems and enzyme receptors. Outside of drug discovery, it factors into ligands for transition metal catalysis, ion-exchange resins, specialty coatings, and corrosion inhibitors. In polymer science, it impacts cross-linking and rheological properties by providing a rigid spacer or reactive node. Each application rewards its combination of chemical inertness, solubility, and the way methylation tunes its behavior compared to simple piperazine.
R&D teams have focused on 1,4-dimethylpiperazine as a template for exploring both biological activity and new material properties. Structural modifications have helped uncover not just potential pharmaceuticals, but also molecules that serve as probes for brain chemistry and potential diagnostics. Materials scientists have looked at it as one knob they can turn to alter polymer structure, harnessing both rigidity and the basic nitrogen atoms for unique macromolecular features. The push for green chemistry means groups continue to seek cleaner routes to the compound and better ways to minimize waste or avoid hazardous methylating agents. More advanced spectroscopic studies keep clarifying how electronic structure and ring dynamics can be tweaked by further substitution on the ring scaffold.
Animal studies and occupational exposure data suggest 1,4-dimethylpiperazine carries an acute toxicity risk on ingestion, inhalation, or skin absorption, though it remains less hazardous than some related amines or halogenated organics. Signs of overexposure can include nausea, headache, or mild neurological symptoms. Long-term carcinogenicity or reproductive effect data remain limited. Standard toxicology tests support labeling as a hazardous chemical in the workplace, though with proper controls and protective gear, incidents stay rare. Wastewater treatment blocks most environmental release, and regulations keep it off the consumer market, restricting it to industrial and lab settings.
Broader adoption of green chemistry frameworks and the steady expansion of chemical biology research both keep 1,4-dimethylpiperazine under active consideration for new roles. Cleaner methylation methods and recyclable catalysts could bring down environmental impact for its production. Biotech and pharmaceutical R&D has yet to truly exhaust its versatility as a scaffold, and materials science finds room to try new derivatives in everything from membranes to sensing devices. With skilled chemists constantly searching for that next breakthrough, familiar molecules with a strong safety record and reliable chemistry often become the foundation for progress. As science pushes further into complex molecular design and sustainable manufacturing, 1,4-dimethylpiperazine still has much to contribute to the next wave of innovation in organic chemistry, materials engineering, and beyond.
Chemicals like 1,4-Dimethylpiperazine rarely grab headlines, but that doesn’t mean they lack impact. I’ve spent enough time around chemical processes to recognize the outsized role specialty compounds play in everyday products. When you hear “1,4-Dimethylpiperazine,” you’re looking at a driven workhorse in organic chemistry circles, not just another line in a formula sheet.
This compound pops up frequently during the search for new medications. Drug researchers often reach for 1,4-Dimethylpiperazine—not because it’s flashy or novel, but because its structure brings stability and flexibility. Many pharmaceuticals require molecules that can “fit” into biological systems, and the structure of 1,4-Dimethylpiperazine helps them do just that. It’s not the main act most of the time, rather a supporting player that helps drugs form and function the way chemists want. Imagine it as scaffolding during the construction of a complicated building.
1,4-Dimethylpiperazine doesn’t just land itself in pharmacy manufacturing. Chemical synthesis often depends on intermediates that can link, protect, or separate other molecules during complex reactions. In my experience—whether tinkering at a university lab bench or walking through a plant floor—you’ll find this compound doing grunt work in agrochemicals, dyes, and specialty resins.
For example, when teams develop a crop-protecting agent, they often require a chemical backbone tough enough for further modifications. Here, 1,4-Dimethylpiperazine stands out, providing the stability to survive several reaction steps. The end-user may never hear its name, but the molecule deserves credit for enabling pest-resistant fields.
Lab analysts rely on predictable reactions, especially when checking purity or setting up quality controls. 1,4-Dimethylpiperazine helps create standardized compounds and reagents. Over the years, I’ve seen this consistency win trust among analytical chemists, resulting in more reliable tests and smoother troubleshooting during research and production scale-ups.
Handling any specialty chemical brings risks. Even reliable workhorses like 1,4-Dimethylpiperazine require proper ventilation, gloves, and training. I’ve seen too many shortcuts lead to headaches—or worse—so emphasizing safety must stay at the forefront of every workplace where chemicals get mixed or stored. Exercising this diligence lines up with broader industry trends: in recent years, regulatory bodies such as OSHA and the European Chemicals Agency have called for stricter labeling, tracking, and emergency planning. These changes improve daily conditions for workers and keep communities near manufacturing facilities safer.
As more companies invest in green alternatives and sustainable chemistry, specialty intermediates like 1,4-Dimethylpiperazine may wind up in new, more eco-friendly applications. Opportunities for reuse and recycling, coupled with better transparency about supply chains, could lessen the footprint of this and similar chemicals.
This isn’t just about chemistry for its own sake; it’s about a behind-the-scenes ingredient supporting safer, smarter innovations that touch lives in ways most never see or think about.
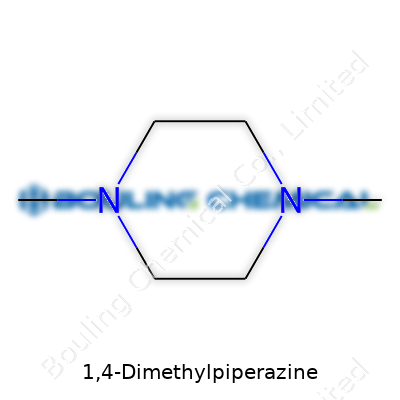
1,4-Dimethylpiperazine catches interest in both research labs and industrial facilities. This compound, with the chemical formula C6H14N2, takes the base structure of piperazine and swaps two hydrogen atoms with methyl groups at the 1 and 4 positions. Imagine a six-membered ring made entirely of carbon and nitrogen atoms—four carbons and two nitrogens. Place methyl groups directly across from each other on the nitrogen atoms and you get a molecule that balances symmetry and reactivity.
If molecular models seem confusing, think of a hexagon with two points representing nitrogen instead of carbon. The rest are basic carbon atoms. Each nitrogen sits opposite the other, or, in chemical terms, at the “1” and “4” positions. Each one wears a methyl group like a hat. Chemically, this setup is written as:
CH3-N-CH2-CH2-N-CH3-CH2-CH2
To show the real structure in two dimensions, chemists often draw the hexagonal ring, mark two nitrogens, attach the methyl groups, and fill in the rest with CH2 linkers. It’s one of those molecules that, despite its relatively simple formula, can lead to a surprising range of applications because of the nitrogen placement.
Over years of working with specialty chemicals, I’ve noticed that the appeal of compounds like this comes from their versatility. Both nitrogen atoms offer lone electron pairs that make the compound act as a base. They’re also perfect sites for further chemical reactions. 1,4-Dimethylpiperazine often becomes a building block for drugs, polymers, and even rust inhibitors. In medicinal chemistry, methyl groups change how a molecule dissolves and how long it sticks around in the bloodstream, so tweaking a piperazine ring by adding methyl groups can shift how a new drug behaves entirely.
Safety forms a big part of the story. Like many compounds that include both carbon and nitrogen, you have to look out for volatility or potential skin irritation. Proper handling and well-maintained ventilation in the lab solve half the problem. For manufacturing, scale-up introduces headaches: reactions involving methylated piperazines tend to create byproducts or require tight controls to keep everything safe and efficient. In my experience, using continuous flow chemistry can cut down worker exposure and cut waste. It doesn’t just protect staff; it keeps processes cost-effective.
Raw material sourcing for piperazine derivatives faces scrutiny now more than ever. The supply chain for the base chemicals touches industries ranging from petrochemicals to agriculture, so improper stewardship carries the risk of disrupting entire sectors. Making sure suppliers follow strict environmental guidelines and choosing those who minimize solvent use alleviates a lot of future headaches. Switching to greener syntheses—shorter reaction times, milder reagents—reduces the indirect footprint of manufacturing. Colleagues in fine chemicals always look for ways to repurpose waste streams or recover solvents, both as a cost saver and a way to show environmental responsibility.
Chemical transparency helps build trust with regulators and customers. Sharing data sheets, risk assessments, and traceability information should be part of any sale—not just a legal afterthought. The more a producer opens up about sourcing and handling, the more likely buyers will come back. As green chemistry guidelines tighten up, the ability to track every step from raw material to finished batch isn’t just nice to have—it’s where the industry is headed. My time in product management backs that up—clear communication and rigorous records have saved projects from disaster more than once.
1,4-Dimethylpiperazine shows up on safety data sheets for a reason. This clear, colorless liquid finds use in chemical research and industry but comes with concerns that deserve attention. Accidental spills, poor handling, or even simple skin contact raise questions beyond just a chemical formula.
Breathing in vapors or letting this compound touch your skin will not feel like a minor mistake. Studies and incident reports point to a real risk of irritation—red eyes, inflamed skin, and sore throats. Eye contact can hurt for hours without washing out the chemical right away. Inhalation causes headaches or a scratchy throat. With repeated exposure or heavy doses, the toll rises. Animal research backs up the evidence that when labs dose test animals with high amounts, the effects move past light irritation.
Mixing or weighing 1,4-dimethylpiperazine without gloves or a mask leads to obvious risks. My experience in academic labs taught me the hard way to never underestimate cheap personal protective equipment. Fumes from heating this chemical hang in the air, triggering coughing fits or dizziness in a closed room.
Some chemicals threaten in more ways than one, and this is the case here. 1,4-dimethylpiperazine can catch fire when exposed to open flames or, sometimes, a simple spark. It forms dangerous mixtures in air, putting both workers and equipment in danger. Once fire starts, fumes turn toxic, especially with nitrogen-containing chemicals like this one, and escaping the lab or plant safely matters more than the lost product.
Accidental mixing with strong oxidizers—a common accident found in the public safety archives—only magnifies the risk. Chemical burns and explosive decompositions enter the picture. Workers and researchers need training that goes beyond watching a single safety video.
Runoff or spills do not end at the workbench. If this compound leaks into the ground or water systems, aquatic creatures land in trouble. Fish and soil microbes react badly, according to EPA hazard databases, which means simple disposal methods fail to cover the problem. Responsible waste management keeps toxins out of drinking water and food chains.
Ignoring basic safety steps can hurt real people. Wearing gloves and goggles, storing in closed containers, and labeling bottles sound routine until one day they save someone’s eyesight. Training matters. Hazard communication boards and routine drills at my workplaces built muscle memory that makes a difference under pressure.
No chemical, no matter how common, should leave workers, students, or neighbors guessing about the risks. Reach out for safety data, demand working fume hoods, and never take shortcuts with cleaning or disposal. Human health and clean water do not come in refillable containers.
Handling chemicals in the lab or on the production floor asks for respect—especially with compounds like 1,4-dimethylpiperazine. This one has a place in pharmaceuticals, chemical synthesis, and specialty applications. Its structure slips under most people's radar, but anyone working near it should keep safety and quality at the top of their minds.
Experience teaches that chemicals with reactive properties don’t forgive sloppy storage. For 1,4-dimethylpiperazine, pick a cool, dry place with plenty of ventilation. Locked cabinets work better than open shelving, and glass or high-grade plastic containers won’t react with the substance. Avoid stacking it near oxidizers, acids, and work gear that might spark a reaction. The chemical likes to draw moisture from the air, so a tightly sealed lid saves you from headaches down the road.
Many older labs learned too late that storing organic liquids near heat sources spells trouble. Even if statistics on thermal decomposition seem remote, fumes or spillage can spread much faster when temperatures creep up. It makes sense to keep storage areas below 25°C and away from any sunlight. UV light and heat tend to speed up breakdown reactions in nitrogen-based organics. By sticking to these basic principles, you limit fire risk and cut down on costly product loss caused by degradation.
Gloves matter more than you think. This chemical has enough volatility to irritate skin and eyes, and repeated contact ups the chance of sensitization. Goggles, proper gloves, and a long-sleeved lab coat go into my routine before lifting a bottle. Anyone who’s ever had a chemical exposure scare probably never forgets how fast things can go wrong. Good ventilation removes vapors from the air. A basic fume hood goes a long way for decanting or mixing. If you’ve seen chemical burns from accidental splashes, taking the extra minute to set up a clean area pays dividends.
Labels get overlooked in busy spaces, but I find that clear, legible tags with product name, concentration, and storage date help everyone. It also helps during inventory checks or audits—no confusion, no mistakes.
Moving 1,4-dimethylpiperazine between sites or rooms calls for more than tossing a bottle in a cart. Secure the cap, wrap it if you’re on rough ground, and use a secondary container—like a plastic carrier—for insurance against knocks or drops. Having spill kits nearby isn’t overkill. I remember a small leak once in transit; quick clean-up with the right spill pillows saved the day and avoided a hazmat call.
Waste disposal rules for this compound demand strict attention. Local regulations shape what counts as safe, but most sites treat it as hazardous waste. Segregate it from incompatible substances and add it to a properly labeled container. Spill response starts with good ventilation and protective gear, followed by absorption with dedicated materials—never with paper towels or rags, which catch fire easily.
Everyone working with chemicals deserves up-to-date training—one outdated habit or skipped step and accidents follow. I’ve seen that sites with routine training have fewer close calls. Review the Safety Data Sheet before your first encounter with 1,4-dimethylpiperazine, even if you feel confident handling similar town-based chemicals. Staying humble and prepared has kept me, and my team, safe for years.
No lab or plant can totally avoid risk, but smart storage, basic protective measures, and routine attention to detail lead to fewer injuries and wasted resources. For 1,4-dimethylpiperazine, extra effort up front means fewer crises later. Chemical handling rewards those who prepare, learn, and stick to proven safety routines.
Anyone who’s worked in a lab or the chemical industry knows the searches don’t start with Amazon or a corner store. 1,4-Dimethylpiperazine is a specialty chemical often used in organic synthesis, pharmaceuticals, and advanced research settings. Plenty of folks might ask where to buy it, but getting your hands on pure, reliable chemicals has layers of difficulty you don’t see with common household goods.
As someone who’s ordered chemicals for research, I always double-check the background of the supplier and the legal hoops involved. 1,4-Dimethylpiperazine isn’t on the list of supermarket finds. Reputable suppliers like Sigma-Aldrich, Thermo Fisher Scientific, TCI America, and Alfa Aesar deal with customers who show credentials. These companies vet buyers to keep dangerous or misused substances out of the wrong hands. You’ll have to prove you represent a business, lab, or educational institution, and some vendors require end-use declarations or compliance documents. Those extra steps come from real concerns about chemical misuse and legal regulation in every country where they sell.
The price for something like 1,4-Dimethylpiperazine doesn’t come with one clear tag. You’ll see prices that fluctuate based on purity, grade, shipping distance, packaging, and—most of all—how much you need. The last time I checked, research-grade 1,4-Dimethylpiperazine sold in the U.S. or Europe ranged from about $70 to $150 for 25g to 100g amounts from top-tier suppliers. Bulk orders often drop the per-gram price, but shipping hazardous materials gets expensive fast. Hazmat fees, international tariffs, and taxes come into play before the chemical even leaves the supplier.
People sometimes look to online marketplaces or obscure international sellers for lower prices. It’s tempting. As someone who’s run into shipping scams and quality issues, I’d urge caution. Poorly handled or counterfeit chemicals can ruin experiments, damage equipment, or worse, cause safety issues. That risk just isn’t worth what you save.
Laws around chemical sales protect public health and the environment. It’s getting harder for independent buyers to get regulated chemicals because governments continue to strengthen rules after high-profile accidents and security incidents. Licenses may be required for purchase or import, and honest mistakes can land you in legal trouble. Anyone considering buying 1,4-Dimethylpiperazine should talk to environmental health and safety officers in their organization before making a purchase. They understand regulatory requirements and how to store or use chemicals safely.
For those in research and development, delays and paperwork can bring frustration. Better pre-screening, faster digital document systems, and clearer online information could make ordering easier for legitimate buyers. Governments and industry should discuss how to streamline verification without lowering standards. Sellers benefit by adding transparency about lead times, purity, certifications, and material safety data sheets on product pages. Most buyers aren’t looking for loopholes—they want to get their work done without headaches or hazards.
Those committed to navigating the world of chemical sourcing do best by building relationships with established suppliers, keeping paperwork in order, and respecting the boundaries that protect everyone. Paying a little more up front pays off through peace of mind and better results in the lab or on the production floor.
| Names | |
| Preferred IUPAC name | 1,4-Dimethylpiperazine |
| Other names |
1,4-Dimethyl-1,4-diazacyclohexane Tetramethylenedimethylpyrazine |
| Pronunciation | /ˈwʌn.fɔːr daɪˈmɛθ.əl pɪp.əˌreɪ.zin/ |
| Identifiers | |
| CAS Number | 106-58-1 |
| 3D model (JSmol) | `3D_jmol_viewer.html?modelid=Mol-0621920251-230-31-6` |
| Beilstein Reference | 1309289 |
| ChEBI | CHEBI:39266 |
| ChEMBL | CHEMBL36833 |
| ChemSpider | 14428 |
| DrugBank | DB08287 |
| ECHA InfoCard | 03abcc3e-3e41-4862-bc71-be52b1e7fd9d |
| EC Number | 208-735-7 |
| Gmelin Reference | 8576 |
| KEGG | C06472 |
| MeSH | D017487 |
| PubChem CID | 6970 |
| RTECS number | TE9275000 |
| UNII | 066TB2S266 |
| UN number | 2269 |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | \("114.19 g/mol"\) |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.857 g/mL at 25 °C (lit.) |
| Solubility in water | soluble |
| log P | 0.16 |
| Vapor pressure | 0.7 mmHg (at 25 °C) |
| Acidity (pKa) | 9.80 |
| Basicity (pKb) | 4.36 |
| Magnetic susceptibility (χ) | -58.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.434 |
| Viscosity | 0.81 mPa·s (20°C) |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 254.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -27.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4493.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Harmful if inhaled. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 62 °C (144 °F; 335 K) |
| Autoignition temperature | 410 °C |
| Explosive limits | 2.2–10.4% |
| Lethal dose or concentration | LD50 (oral, rat): 2640 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 269 mg/kg |
| NIOSH | DSG857 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | IDLH: 100 ppm |
| Related compounds | |
| Related compounds |
1,2-Dimethylpiperazine 1,3-Dimethylpiperazine Piperazine 2-Methylpiperazine 1-Methylpiperazine |