Trawling through the history of chemical synthesis shows the evolution of 1-(3-Methoxypropyl)-4-piperidinamine stems from both academic curiosity and industrial urgency. The molecular backbone links a piperidine ring, a well-known structure since the late 19th century, with a methoxypropyl chain to create flexibility for medicinal research. Early explorations in piperidine chemistry, particularly post-World War 2, built the foundation for modern day functional group manipulation. As researchers sought new routes for central nervous system agents, adding a 3-methoxypropyl group to 4-piperidinamine grew from trial-and-error into a strategic step for tuning pharmacological activity. Watching the shift toward structure-activity relationships, chemists in both universities and industry lab benches shaped the path that led directly to this compound. This agent shows how the heritage of organic chemistry always calls back to people taking modest steps, testing new links, jotting down spectral data, and noting down points where things turn unexpectedly productive.
1-(3-Methoxypropyl)-4-piperidinamine has found its way into reagent shelves and medicinal chemistry screens. Scientists eye this molecule for both its core and side chain. Its piperidine ring offers stable nitrogen heterocycle chemistry, while the 3-methoxypropyl allows for both solubilizing and fine-tuning electronic properties. Designed for versatility, this compound turns up as both an intermediate and a template for further research. Use cases often range from building blocks in fine chemical synthesis to niche roles in active pharmaceutical ingredient (API) studies. It's common to spot this chemical in synthetic routes where improved CNS penetration or cell permeability is required, making it particularly relevant to drug discovery programs. Real-world utility depends on reliable supply, clear documentation, and enough flexibility for researchers to test modifications and run targeted assays.
Looking at the substance itself, 1-(3-Methoxypropyl)-4-piperidinamine typically appears as a colorless to slightly yellowish liquid under standard conditions, offering moderate water solubility because of the amine and ether functionalities. The presence of the methoxy group often lends it a slightly sweet odor, familiar to organic chemists. Boiling points settle around the mid-to-high range for small-molecule amines, matchable with its moderate molecular weight. The amine and ether functionalities bring good compatibility with both aqueous and organic solvents, supporting diverse applications. Thermal stability meets industrial handling expectations, but standard storage calls for cool, dry conditions to minimize degradation or unintended side reactions. Understanding these characteristics matters not because they sound impressive, but because they impact how easily a synthetic chemist can work up, extract, purify, and store products in day-to-day routines.
Vendors usually label 1-(3-Methoxypropyl)-4-piperidinamine by its systematic name, with CAS number, purity by GC or HPLC (commonly exceeding 97% for research-grade samples), physical form, batch number, and storage instructions. Labels also warn about basic hazards such as skin or eye irritation, reflecting amine reactivity. Researchers learn to check certificate of analysis not out of red tape, but to prevent wasted time chasing an impure or illegibly labeled batch. Safety Data Sheets spell out its handling requirements, emphasizing ventilation and proper barrier protection. Clear technical documentation, batch traceability, and supply chain verification ensure quality control, especially as pharmaceutical labs comply with ever-tightening regulatory standards.
Synthesizing 1-(3-Methoxypropyl)-4-piperidinamine usually starts with piperidine chemistry. A common route involves alkylation of 4-piperidinamine with 3-methoxypropyl halide under controlled conditions. Chemists pick solvents like acetonitrile or DMF for good solubility; base like potassium carbonate helps drive the nucleophilic substitution. Reaction times vary, but monitoring by TLC or GC guides end-point determination. Post-reaction cleanup relies on aqueous extraction, followed by distillation or chromatography to isolate the product. Residual base, side products, and unreacted starting materials require careful removal, as traces can jeopardize downstream applications. Real-world synthesis rarely goes as cleanly as a textbook promises, so every step, from scale-up troubleshooting to recrystallization tweaks, adds valuable lessons.
1-(3-Methoxypropyl)-4-piperidinamine stands ready for a wide range of transformations. Its amine group welcomes both acylation reactions and reductive aminations, making it attractive for conjugating to pharmacophores or linking units. Functionalization of the methoxy group, such as O-demethylation or conversion to higher-order ethers, expands the chemical space for SAR studies. The piperidine ring, robust as always, holds up under conditions for selective oxidation, aromatic substitutions, or cyclization cascades. Such flexibility keeps this compound in rotation on research benches, as scientists push for small tweaks that might yield better biological properties or fine-tuned pharmacokinetics. Watching a structure go from a flat line drawing to a tailored intermediate reminds us that every molecule is an opportunity for inventive improvements.
Across catalogs and literature, alternative names for 1-(3-Methoxypropyl)-4-piperidinamine sometimes cause confusion. Chemists call it N-(3-methoxypropyl)piperidin-4-amine or 3-methoxypropyl-(4-piperidinyl)amine. Trade listings sometimes abbreviate the compound or tuck it under “intermediate B-524.” These differences in naming underscore the real need for clear, agreed-on terminology, especially as compounds slip between research papers, supplier catalogs, and regulatory filings. Getting names right lowers the risk of mix-ups and missed hits in chemical databases.
Working safely with 1-(3-methoxypropyl)-4-piperidinamine means more than gloves and goggles. Small-molecule amines can sting eyes and skin, so fume hood use comes standard practice. Proper waste disposal matters, since amine-containing compounds can smell strong and react with oxidizers. Review of safety data points to irritation as a main risk, with little evidence for more severe effects under typical conditions, but careless spills or splashes cause problems if not managed quickly. Labs handling this reagent adopt robust ventilation and label every storage vessel, staying ready for accidents with spill kits and first-aid. Repeated safety audits, updated protocols, and internal training all reinforce a culture where routine work stays predictable and reliable, protecting both people and research investments.
Most interest in 1-(3-methoxypropyl)-4-piperidinamine comes from its use in medicinal chemistry. Its molecular framework appears in candidate structures for CNS drugs, small-molecule receptor modulators, and next-generation antihypertensives. In academic labs, it acts as a building block for libraries screened against neurological, metabolic, or infectious targets. Some researchers modify the side-chain to study how methoxy versus hydroxypropyl or ethoxypropyl shifts bioavailability or activity. Out beyond pharma, the compound finds use in custom organic synthesis routes or specialty materials research, thanks to its modifiable nature and relatively low toxicity. Real-world use never stays static; every year brings fresh literature chasing tweaks that might lead to the next hit compound or a more sustainable synthesis route.
The research community’s focus on 1-(3-methoxypropyl)-4-piperidinamine depends on opportunities for meaningful chemical innovation. In pharmaceutical pipelines, this backbone serves as a launchpad for structure-activity relationship investigations, where variances in chain length, aromaticity, or branching give insight into molecular recognition. Medicinal chemists draw from enrolled clinical trial data, computational docking, and bench assay work to guide modifications. This way, the compound becomes not just a reagent, but a node in a web of discovery. Publications in peer-reviewed journals chronicle modifications, new synthetic methods, or analytical toolkits for quality control. Collaboration between universities and industrial R&D shops keeps new uses emerging, from prodrug strategies to linker chemistry in antibody-drug conjugates. Productivity rises not just with new grants or fancier equipment, but with the willingness to re-examine old compounds for innovative twists.
Laboratory safety reviews, animal model screenings, and computational modeling shape our current knowledge of 1-(3-methoxypropyl)-4-piperidinamine's toxicity profile. The structure—the piperidine ring and alkyl side chain—usually raises only moderate concerns. Routine in vitro data point toward limited cytotoxicity at concentrations used for research. Reports on skin and mucous membrane irritation match typical primary-amine effects, suggesting reasonable safety margins for laboratory use. Regulatory trends aim for more comprehensive in vivo testing, echoing public demand for responsible disclosure. Some labs now add environmental persistence and bioaccumulation studies, driven by the larger push for green chemistry and risk management. Toxicologists keep refining predictive models, but hands-on handling still rests on treating the chemical with healthy respect.
The outlook for 1-(3-methoxypropyl)-4-piperidinamine looks promising—not just as a chemical tool on a shelf, but as a stepping stone in drug and material science. Synthetic access continues to improve, with greener processes and fewer hazardous reagents moving from theoretical papers into reliable practice. Pharmaceutical researchers hunt for faster, more modular modifications as drug discovery grows ever more data-driven. Expansion into new therapeutic areas, or adaptations for delivery via advanced formulations, stay on the near horizon. Environmental and occupational safety teams press for lower-impact production cycles, tapping into both regulatory necessity and the ethical drive felt by modern chemists. Looking ahead, those who work with this compound will shape its next chapters through persistence, curiosity, and a willingness to solve practical problems, as chemical science keeps moving from bench to bedside and back again.
Science classrooms and pharmaceutical labs always buzz with compounds carrying hard-to-pronounce names. 1-(3-Methoxypropyl)-4-Piperidinamine comes up often in chemical catalogs but doesn’t usually grab the headlines. In plain terms, this compound’s structure connects the familiar piperidine ring—a backbone in many drugs—to a methoxypropyl side chain. It’s a raw material, a building block, as people in the field like to say. My past research in medicinal chemistry circles involved plenty of similar molecules, so its presence rarely surprised me.
Many years on the research bench taught me the value of compounds like this one. Researchers harness it during the early stages of drug synthesis, tweaking its structure to scout for new medicines. That piperidine core keeps popping up in medicines ranging from allergy relief to advanced painkillers. 1-(3-Methoxypropyl)-4-Piperidinamine slots easily into chemical reactions that produce potential drug candidates. Sometimes, even a small tweak to a side chain can mean the difference between a compound that helps and another that’s completely useless in human cells.
Lab suppliers ship this compound out in small to medium quantities. Chemical engineers and synthetic chemists value its clean reactivity. It fits into the synthesis of specialty polymers and serves as a stepping stone toward more complex molecules. Researchers in government agencies and universities use it to probe new chemical space—a process always full of trial and error.
In industrial settings, folks focus on finding efficient paths to build larger products. 1-(3-Methoxypropyl)-4-Piperidinamine steps into this role as a flexible part of the puzzle. For example, chemists can attach fluorescent tags or protective groups to its amine, leading to tools for studying diseases at a cellular level. Its core structure appears in some advanced adhesives and coatings as well, mostly behind the scenes in R&D labs.
Handling specialty chemicals comes with a tight set of responsibilities. Labs require strong documentation and traceability, especially if a substance looks similar to ones used in the broader pharmaceutical or agrochemical trades. My own work always demanded a double-check from both safety and regulatory staff. Accidental misuse, even if rare, can bring unwanted scrutiny. 1-(3-Methoxypropyl)-4-Piperidinamine doesn’t carry the baggage of high-profile toxins or addictive drugs, but the industry approach always leans toward caution.
Research keeps marching forward, finding new use cases for old compounds. Too many promising drug candidates fail in trials, but the creative work done with building blocks such as this one drives the field ahead. Sharing data between labs, investing in safer synthetic routes, and upskilling researchers form the front lines of responsible progress. In my own experience, sharing protocol tweaks or results, even after a failed experiment, helped colleagues avoid dead ends and uncover smarter approaches.
Chemicals like 1-(3-Methoxypropyl)-4-Piperidinamine rarely earn the spotlight, yet their impact ripples through every new innovation in drug discovery and specialty materials. Staying grounded, keeping safety front and center, and promoting collaboration will support not only reliable results but also public trust in the industry’s efforts. The story of progress in laboratories—a story I’ve seen firsthand—relies just as much on these quiet contributors as it does on blockbuster discoveries.
Organic chemistry isn’t always about mixing things and hoping for fireworks. Sometimes, it’s about picking through names and teasing out what those names mean on a structural level. Take 1-(3-Methoxypropyl)-4-piperidinamine. The name points right away to a familiar backbone—piperidine, the six-membered ring with that tell-tale nitrogen atom. Substitutions tell most of the story.
Let’s look at the “4-piperidinamine” part first. A piperidine ring has five carbons and one nitrogen. Add an amino group at the fourth position. Move up the name to that extra-long group: “1-(3-Methoxypropyl).” Here, at the “1” position on piperidine, a three-carbon chain comes off, ending in a methoxy group. That’s an -OCH3 tacked on the tail-end of a propyl chain.
Long ago, my mentor taught me to start counting at the last functional group. Point by point, this molecule wraps together at the core—one piperidine ring (C5H11N). The 3-methoxypropyl arm adds three more carbons, one more oxygen, and another three hydrogens for the methoxy. The amine at the 4-position adds an NH2 group.
Now, count the totals: 8 carbons (5+3), 20 hydrogens (11+7+2), 2 nitrogens (1 from the ring, 1 from the amine), and 1 oxygen. The sum leads you to C8H20N2O.
The devil’s in the details, especially in drug design and material science. Molecular formulas give away much more than atom counts. I remember my time in the lab, puzzling over samples with nearly identical masses but strikingly different effects—a methyl group out of place, a nitrogen where an oxygen belonged. These small distinctions separated a painkiller from a poison, an industrial solvent from a plasticizer.
Put two compounds side by side; both may aim for the same target, but only the right arrangement unlocks the effect chemists want. Structure controls solubility, reactivity, and biological activity. Research shows that molecules like substituted piperidines play huge roles in pharmaceuticals, finding their way into antipsychotics and antihistamines. Universities and chemical suppliers carry catalogs bursting with these variants, each one telling its story though a slight change in formula.
One overlooked risk with tricky molecular names: subtle mistakes in reading add up fast. Mixing up the direction of a chain or counting the amine at the wrong position leads to completely different recipes. Accuracy doesn’t just help build the right molecules—it keeps people safe. Laboratories lose resources and time with every misstep.
Adopting checklists in the lab and using structure-drawing software cuts down on error. Open-access databases let chemists double-check their work—critical for students and veterans alike. The top solution lies in education: keep organic chemistry grounded in real analysis, not rote memorization of patterns. Groups like the American Chemical Society push for hands-on projects, giving students muscle memory in piecing these things together.
Even a simple question like the molecular formula of 1-(3-methoxypropyl)-4-piperidinamine uncovers the depth behind structural chemistry. For anyone who crafts molecules, getting it right is more than a detail—it's the foundation for everything that follows. The formula C8H20N2O captures not just atomic counts, but the story of a specific shape in the endless world of organic molecules.
1-(3-Methoxypropyl)-4-Piperidinamine doesn’t show up on a list of household products, but chemicals like it often turn up in industrial labs, research facilities, and chemical supply chains. The questions start popping up as soon as a long chemical name enters the scene. Is it dangerous? Do regular people, workers, or even scientists need to treat it with extra care?
Occupational safety around new and lesser-known compounds means chemists and lab workers depend on data sheets, peer-reviewed toxicology studies, and sometimes even the stories of others who’ve handled the substance. Data on this specific compound remains limited. It’s not as famous or widely used as some of the solvents or reagents taught in undergrad chemistry, but its backbone—piperidine—comes from a family of chemicals often linked with irritant effects. Amines and related compounds can trigger headaches, skin or respiratory irritation, and, in worse cases, systemic toxicity. Throw in the methoxypropyl part, which signals a potential for unusual reactivity.
If someone has spent time around amine compounds, they’ve learned to respect even trace exposure—burning sensations, nausea, or red skin turn up fast if gloves aren’t tight or a fume hood isn’t running. The European Chemicals Agency and the US EPA maintain databases of similar piperidine or alkoxy-substituted compounds. Patterns emerge: skin absorption, eye corrosion risk, odor that chokes up a room. A study published in the Journal of Occupational Medicine details how a related class caused persistent dermatitis in lab techs after repeated short-term skin contact.
On a personal level, handling comparable amines taught me never to put faith in just a lab coat and goggles. Tiny drops spilled on a sleeve can seep through in minutes. A face mask might not block vapor that stings the nose. Replacement gloves matter: disposable latex fails with more aggressive amines, while nitrile gives better, though not infallible, protection. Chemical fume hoods serve as the workplace’s best insurance. If a smell breaks through, that usually means airborne levels outpace what ventilation can handle.
Even if the MSDS for 1-(3-Methoxypropyl)-4-Piperidinamine hasn’t spelled out every danger, experience with its family tree makes hazard control plain. Engineering controls, sturdy PPE, and solid training keep danger in check. Ignoring the gear, or taking shortcuts, leads to proven cases of chemical burns, allergic reactions, and sometimes long-term lung damage from inhaled vapors. A report from the CDC underscores how easy it becomes to underestimate cumulative effects if early symptoms—dizziness, red eyes, skin tingling—get shrugged off.
Protocols ought to evolve based on the “unknowns.” Companies should support safety data requests, encourage reporting of even minor exposures, and phase in substitutes if toxicity data tallies up greater than expected risk. Open lab meetings, clear incident reporting, and regular reviews of chemical inventories keep hazards visible. The ECHA recommends annual refresher training and full incident follow-ups for this category of amine. Trust grows not just in manuals and chemical lists, but in workers who speak up and share lessons learned—before any lasting harm settles in.
Long chemical names rarely grab headlines, yet each carries behind it a story of uncertainty or, occasionally, harm. Keeping harm at bay requires an honest look at data, an ear for those already handling the stuff, and a little humility about nature’s complexity. Even if 1-(3-Methoxypropyl)-4-Piperidinamine never becomes a mainstay, its risks—and the smart ways people tackle them—echo across every modern chemical workplace.
1-(3-Methoxypropyl)-4-Piperidinamine isn’t the sort of stuff you leave lying on an open shelf. Labs and chemical users handle it with extra care because this compound, like many amines, shows sensitivity to both air and moisture. Keep it away from heat sources, sparks, or anything that could start a fire. This isn’t just good practice—mistakes here often lead to more than a ruined experiment.
From my days in the lab, I learned how moisture loves to sneak in where you least expect it, especially with hygroscopic compounds. Letting even a small amount of water vapor into the container can lower purity, spoil your batch, and play havoc with results. Silica gel packs or a fresh desiccant help a lot here. Tightly closed containers beat makeshift lids every time.
Store this chemical in a cool, dry place, away from direct sunlight. Heat in a lab sometimes feels like an afterthought until a thermometer shows the wrong side of 25°C. Chemicals like 1-(3-Methoxypropyl)-4-Piperidinamine can break down faster under warm conditions, so manufacturers recommend sticking to the lower end of room temperature, around 2–8°C if possible. Standard laboratory refrigerators usually do the job, especially if you label your bottles clearly and don’t stack incompatible substances together.
Open air can bring more than just moisture. Oxygen likes to react with amines, sometimes creating compounds that cause allergies or are toxic. Keep those lids screwed tight. If someone forgets and leaves a bottle uncapped, it’s not just their narrow miss—everyone in the area gets put at risk. Small mistakes in the name of convenience almost always turn into bigger headaches. That’s why I’ve always slid a reminder label right onto the bottle: “Seal tightly after use.” Before you know it, this becomes routine for everyone handling lab chemicals.
I’ve seen mislabeling accidents turn into confusion about what’s actually inside a jar. Write the chemical name, date of receipt, and expiry date right on the container, clear and big. Sure, it takes a minute, but it beats confusion. Store it separate from acids and oxidizers—combine them and you could end up with dangerous reactions. I remember a story from a colleague who learned that lesson the hard way and trust me, no one wants to air out an entire wing of a building after a spill.
Original packaging almost always beats anything improvised. Manufacturers pick bottles for a reason. If switching containers becomes necessary, pick clean, chemical-resistant bottles made out of glass or thick, high-density polyethylene. Always use fresh, dry spatulas or syringes to withdraw the compound; avoid dipping a used tool between multiple bottles.
Old or contaminated 1-(3-Methoxypropyl)-4-Piperidinamine isn’t just thrown out with regular garbage. Local regulations usually demand professional waste contractors for this type of chemical. Keeping an up-to-date inventory helps manage disposal cycles efficiently, and that keeps everyone safe in the long run.
Consistent attention to air-tight containers, clear labels, dryness, and safe temperature pays off every time. It’s not about ticking boxes—it’s about lowering risk and keeping people and projects on track. Safety culture doesn’t come from a manual; it grows through small habits and shortcuts avoided. 1-(3-Methoxypropyl)-4-Piperidinamine needs the same respect as any high-stakes chemical. Give it that, and both projects and people stay protected.
Every chemist and quality manager who has witnessed a project delayed by contaminated starting materials knows the truth: the specs behind your chemical matter more than the product brochure. Purity specifications for 1-(3-Methoxypropyl)-4-Piperidinamine don't just signal a lab’s discipline, they shape safe results in both research and industry work.
For 1-(3-Methoxypropyl)-4-Piperidinamine, most credible suppliers guarantee a minimum purity of 98%, based on high-performance liquid chromatography (HPLC) or gas chromatography (GC) data. Pharmaceutical and biotech labs often push requirements above this threshold if their application leaves little room for error. Trace levels of water, heavy metals, or unreacted starting materials also get attention, particularly if the amine ends up as a key building block for active molecules.
It’s easy to say 98% or 99% sounds near-perfect, but those decimal points hide real risks. Synthetic routes can struggle if a chemical introduces stray byproducts. I've seen unstable intermediates pop up out of nowhere because someone trusted a “technical” grade product that only looked right on paper. A missed impurity can translate to hours down the drain — or worse, an entire batch lost.
Transparency around a compound’s purity impacts downstream results and, by extension, patient safety or product reliability. Pushing for the highest grade isn’t about snobbery. A data sheet should show not just the bulk purity, but individual impurity profiles, solvent residues, and any remaining unreacted amines or byproducts. Some labs want to see levels for metals like iron or copper, even when the main job is simple amine alkylation. These metals, if unchecked, can open the door to catalyzed side-reactions or cytotoxicity in biomedical work.
Not all suppliers play at the same level. Reliable documentation, a batch-specific Certificate of Analysis, and the willingness to share analytical spectra all reflect trust and expertise. If a supplier dodges those requests, it’s a red flag. Anyone serious about the science checks for these every time, especially if using 1-(3-Methoxypropyl)-4-Piperidinamine for new drug development or critical intermediates.
Most headaches over purity start at sourcing. Direct relationships with reputable suppliers—or even in-house testing—make a world of difference. Labs should consider running short pilot reactions, not just trusting paperwork. Thin-layer chromatography (TLC), HPLC, or even simple melting point checks give early warnings before scale-up. I once caught a supplier’s slip just by sniffing out a faint, off note—sometimes experience trumps brochures.
Handling and storage should match the sensitivity of the chemical. Sealed containers, minimal light exposure, and proper drying agents cut down on degradation or atmospheric moisture. Even top-grade product can lose purity on a humid shelf or in careless hands. Training your team, labeling everything, and keeping a clean logbook mean more than any shiny packaging.
The end-user’s trust—whether it’s a researcher, a patient, or a regulator—relies on details. 1-(3-Methoxypropyl)-4-Piperidinamine might seem far removed from the final medicine, but if the base isn’t pure, the whole stack of results loses ground. Good habits in chemical sourcing and quality checks end up saving projects, budgets, and reputations.
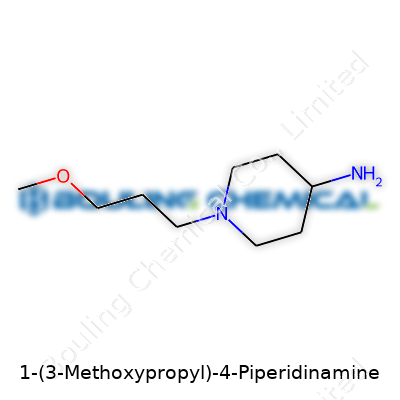
| Names | |
| Preferred IUPAC name | 4-(3-Methoxypropyl)piperidin-1-amine |
| Other names |
1-(3-Methoxypropyl)piperidin-4-amine 4-Amino-1-(3-methoxypropyl)piperidine |
| Pronunciation | /wan-θriː-mɛθˈɒksiprəʊpɪl-faɪˈpɪrɪdɪnəˌmiːn/ |
| Identifiers | |
| CAS Number | 138176-04-0 |
| 3D model (JSmol) | `load data:text/plain,3Dmol; structure=CCCCN1CCC(CC1)N` |
| Beilstein Reference | 3112965 |
| ChEBI | CHEBI:189880 |
| ChEMBL | CHEMBL2111090 |
| ChemSpider | 21434915 |
| DrugBank | DB08375 |
| ECHA InfoCard | 01d2d115-cd97-4b4b-9ba3-f1fac1cacda5 |
| EC Number | 620-505-6 |
| Gmelin Reference | 79530 |
| KEGG | C19602 |
| MeSH | D058965 |
| PubChem CID | 160684 |
| RTECS number | TJ8575000 |
| UNII | 75J6T1O9ZA |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C9H20N2O |
| Molar mass | 188.28 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Odorless |
| Density | 0.997 g/cm3 |
| Solubility in water | soluble |
| log P | 0.0 |
| Vapor pressure | 0.2 mmHg (25 °C) |
| Acidity (pKa) | 11.1 |
| Basicity (pKb) | 4.03 |
| Magnetic susceptibility (χ) | -72.4 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.495 |
| Viscosity | Viscosity: 6.6 cP (Predicted) |
| Dipole moment | 3.25 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 230.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4835 kJ/mol |
| Pharmacology | |
| ATC code | N06AX22 |
| Hazards | |
| Main hazards | Harmful if swallowed or in contact with skin. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P264, P270, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 118°C |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
1-(3-Hydroxypropyl)-4-piperidinamine 1-(3-Chloropropyl)-4-piperidinamine 1-(3-Bromopropyl)-4-piperidinamine 1-(3-Propoxypropyl)-4-piperidinamine 1-Propyl-4-piperidinamine 1-(2-Methoxyethyl)-4-piperidinamine |