The story of 1-(3-Chloropropyl)-4-methylpiperazine tracks the growth of modern organic chemistry. Researchers in the late 20th century explored piperazine and its derivatives for their reactivity and compatibility with a wide range of reactions. Early investigations blended practical lab work with the search for new pharmaceutical scaffolds, pesticides, and specialty chemicals. Developing new alkylated piperazines responded to the need for functional groups that improved stability and performance. 1-(3-Chloropropyl)-4-methylpiperazine emerged from that search, gaining traction among synthetic chemists searching for a reliable intermediate.
Manufacturers produce 1-(3-Chloropropyl)-4-methylpiperazine as a building block in the synthesis of drugs, agrochemicals, and selective organic ligands. Its structure supports tuning for specific chemical properties. The methylpiperazine core with a chloropropyl sidechain grants flexibility, improved solubility, and controlled reactivity. Companies valued this intermediate for its shelf stability and predictable performance in further modifications, helping expand its market beyond fine chemicals to pharmaceuticals and research supplies.
This compound usually appears as a clear, slightly yellowish liquid or low-melting solid. Boiling point hovers just above 100°C under reduced pressure. Density hovers around 1.02 g/cm³, and the compound dissolves well in polar organic solvents, including ethanol, methanol, and dichloromethane. It displays modest volatility and resists hydrolysis under mild conditions. The chloropropyl functionality stands out as a reactive site for nucleophilic substitution, while the methyl group on the piperazine ring improves steric protection and slightly bumps up the compound’s lipophilicity, which comes in handy in medicinal chemistry projects aiming for better membrane permeability.
Companies set tight standards for purity, water content, and trace metals. Typical purity runs above 97%, with GC or HPLC as the analytical touchstone. Lead and heavy metal limits sit below 10 ppm to minimize contamination risk during downstream synthesis. Labels on containers highlight hazards—skin and eye irritation warnings, flammability data, and requirements for storage in well-ventilated, dry environments. Details like batch number, manufacture date, and expiration ensure traceability, which helps support both regulatory compliance and research reproducibility.
Most producers synthesize 1-(3-Chloropropyl)-4-methylpiperazine from 4-methylpiperazine and 1,3-dichloropropane under basic conditions such as potassium carbonate in a polar aprotic solvent. They rely on a straightforward N-alkylation, then purify by distillation or extraction. Some labs swap in tosylated intermediates for a gentler approach, trading off longer reaction time for cleaner conversion and improved yields. Optimizing temperature, reaction time, and molar ratios can push the process toward higher selectivity and reduce unwanted byproducts. Lab techs need to watch the reaction closely, since even small deviations can lead to persistent impurities that complicate downstream processing.
Chemists value the chloropropyl group for its flexibility. It reacts smoothly with nucleophiles—amines, thiols, or alkoxides—to make more complex molecules. Substitution reactions build new C-N, C-S, or C-O bonds, making the compound a springboard to many functionalized derivatives. The piperazine ring can also take on further substitution or acylation without disrupting the original framework. These transformation options power the material’s popularity as a linker or core in the synthesis of bioactive compounds, dyes, or coordination polymers.
Industry and science circles know this compound by several names, including 3-chloropropyl-N-methylpiperazine, N-methyl-N-(3-chloropropyl)piperazine, and 1-methyl-4-(3-chloropropyl)piperazine. Some catalogues use product codes unique to distributor or regulatory tracking. Synonym confusion can trip up even experienced researchers, so double-checking CAS numbers and chemical structures stays essential before ordering or reporting data.
Laboratory work with 1-(3-Chloropropyl)-4-methylpiperazine means gloves, goggles, and chemical fume hoods. Skin or eye contact produces persistent irritation, and inhalation risks linger for those who neglect proper ventilation. Safety data sheets call out the substance’s environmental hazards in case of spills, and recommend containment, neutralization, and responsible solvent disposal. Firms must train staff on correct storage: cool, dry places away from acids and strong oxidizers. Emergency showers and eyewash stations anchor operational safety, and routine audits keep everyone sharp on protocols and documentation to satisfy OSHA, REACH, and local regulators.
Many labs use this intermediate to stitch together active pharmaceutical ingredients, particularly those related to central nervous system drugs or anti-infectives. Agrochemical companies see potential for herbicide and insecticide candidates, as the piperazine structure aligns with known bioactivity patterns. Development programs in specialty polymers and advanced materials sometimes tap it for its dual nucleophilic and electrophilic modes, allowing dense crosslinking or precise functionalization. Medicinal chemists appreciate starting with a versatile, reliable intermediate so molecular innovations can move ahead without nagging supply concerns.
R&D teams in industry and academia continue to study improved synthetic routes, looking for greener solvents and milder conditions. Automation and flow chemistry research seek safer, higher-throughput preparation. Some scientists target selective activation of the chloropropyl chain, using catalysts to favor mono-substitution or regioselective branching. These innovations matter because regulatory bodies now expect lower waste, tighter purity, and detailed impurity profiles in drug development. Novel applications keep popping up over the last decade, including links to nanomaterials and stimuli-responsive molecular systems, especially as researchers recognize the value of heterocyclic backbones in modern material science.
Systematic data on acute and chronic toxicity remain limited, though preliminary evidence flags this compound as a moderate irritant. Researchers caution against prolonged exposure and urge careful monitoring due to the presence of the alkyl chloride group—these are known as potential alkylating agents that can pose DNA reactivity risks. Some toxicity studies look at environmental breakdown, with findings pointing toward modest persistence and the potential for metabolite build-up. Teams assessing occupational hazards rely on animal models and in vitro systems, pushing for more in-depth studies as use cases grow in pharma and agro-chemistry. Responsible handling, hazard communication, and medical surveillance back up safe lab practices.
Research shows demand isn’t slowing as piperazine derivatives fuel drug discovery and materials innovation. Synthetic tweaks and advances in green chemistry will keep reshaping how chemists approach both production and product development. Regulatory scrutiny only tightens over time—producers and buyers need well-documented quality control and ever-stricter environmental stewardship. The flexibility of 1-(3-Chloropropyl)-4-methylpiperazine means it will keep finding new life in unexpected industrial, academic, and interdisciplinary projects. For those in the trenches of chemical synthesis, this intermediate serves not just as a tool, but as a case study in the long-term value of robustness, adaptability, and clear-eyed safety thinking.
Spend any time in a laboratory, and you’ll see chemical bottles lined up with names only a chemist could love. One such name, 1-(3-Chloropropyl)-4-Methylpiperazine, shows up quietly on order forms and research papers. Few outside of pharmaceutical chemistry have come across it, yet this compound plays a bigger part in drug research than most people realize.
The pharmaceutical industry often relies on chemicals like this one to construct more complex molecules. Over the years, I’ve seen researchers use 1-(3-Chloropropyl)-4-Methylpiperazine as a core ingredient when creating experimental treatments. Its structure offers a reliable starting point for building other compounds, particularly when developing drugs aimed at conditions affecting the brain—think antidepressants, antipsychotics, or drugs for neurodegenerative diseases.
Why has this substance found a home in so many drug labs? It comes down to its chemical shape and the specific ways it links with other compounds. Doctors and researchers always look for ways to fine-tune how drugs interact with brain receptors. The piperazine ring at the center of this molecule helps anchor new creations, giving scientists the skeleton they need to design focused, active medicines. Several existing drugs trace their roots back to similar building blocks.
Sitting with chemists over coffee, I’ve learned how important safe handling remains for compounds like 1-(3-Chloropropyl)-4-Methylpiperazine. Its chemical structure, while useful, means it can also be toxic or cause reactions if not respected. Many labs I’ve visited keep a close eye on ventilation, gloves, and proper waste disposal. Mistakes can cost years of hard work, not to mention risks to health.
Rigorous training around such compounds helps prevent surprises. Institutions investing in safety and education often see better research results and healthier employees. Open conversations about new chemicals—and potential hazards—involve everyone from entry-level techs to research directors.
Because chemicals like this have more than one use, responsible sourcing and documentation have gained attention. Regulations exist, though they change country by country. Pharmaceutical firms need clear records from suppliers, not just to stay inside the law but also to protect the integrity of scientific work. Counterfeit or low-purity chemicals can undo years of progress, or worse, introduce harmful contaminants into research.
Responsible companies check suppliers, invest in quality control testing, and keep up with legal requirements. I’ve seen projects saved by double-checking, catching impurities that would have turned promising results to dust. Younger researchers benefit from mentorship here, learning not to cut corners even when deadlines loom.
Some people might scoff: Why care about another arcane laboratory chemical? In my view, every new drug on the pharmacy shelf began with decisions about molecules most would never notice. 1-(3-Chloropropyl)-4-Methylpiperazine turns up quietly, but it allows for creative problem-solving in medicine. This building block gives researchers the control they need to tweak, adjust, and one day perhaps deliver new hope for patients facing tough diagnoses.
Progress in science doesn’t always come from headline-grabbing breakthroughs. Sometimes, it grows out of smart choices and quiet work with safe, well-chosen ingredients—just like this one. Careful use and continuous oversight mean the real promise of chemicals like 1-(3-Chloropropyl)-4-Methylpiperazine can be realized, one careful step at a time.
Most chemists and researchers want facts right away when looking up any compound. 1-(3-Chloropropyl)-4-methylpiperazine comes with the chemical formula C8H17ClN2. Spend enough time in a lab and you get used to memorizing strings like this — each shorthand packed with practical relevance. Its molecular weight clocks in at 176.69 g/mol, which matters a lot more than it might seem at first glance.
Let’s get real about what that actually means. The chemical formula gives more than just a label. With C8H17ClN2, you are looking at an organic molecule with a piperazine core. Throw in a methyl group and a chloropropyl chain — and you land on a compound that can wander into several directions in organic synthesis. The extra chlorine atom is no trivial add-on; it influences reactivity, solubility, and even the way this molecule interacts during pharmaceutical development or in chemical manufacturing. Countless projects hang on subtle differences like these.
Molecular weight affects everything from dosage to solvent choice and even shelf-life in storage. At 176.69 g/mol, researchers can figure out how to prepare solutions, calculate yields, and anticipate reaction outcomes. If you’ve ever stood over a scale measuring out a new reagent for a test batch, these calculations aren’t distant academic trivia. They shape every step, sometimes down to the milligram.
Real-world chemistry brings real-world consequences. Compounds like 1-(3-chloropropyl)-4-methylpiperazine aren’t found on household shelves, and that’s a good thing. Having that chlorine attached means certain precautions come into play. Gloves, goggles, well-ventilated spaces, and a clear understanding of safety data sheets go without saying. If you skip safety out of habit or rush, it only takes one incident to set you straight. Before long, everyone in the lab has a story to tell about a splash or spill that forced a rethink of their process.
The regulatory world zeros in on these compounds for a reason. Rich in nitrogen, with that methyl group and the chlorinated chain, this molecule has caught the eye of pharma researchers searching for new treatments. At the same time, illicit labs sometimes chase chemical cousins for entirely different reasons, so tracking and tracing such building blocks becomes a public health issue. Legal frameworks and supply chain transparency aren’t just red tape; they keep dangerous situations at bay and protect intellectual property for legitimate work.
Every time a chemist asks for a chemical formula or molecular weight, it signals a moment of planning, research, or synthesis that could lead somewhere new. Mistakes can be costly, so accuracy underpins everything. For those new to the world of chemical synthesis, understanding these fundamentals and thinking proactively about safety can transform how people work. For more experienced hands, details like C8H17ClN2 and 176.69 g/mol guide collaboration, troubleshooting, and innovation. The right details, checked and cross-checked, become the first step toward better results and safer outcomes, setting the stage for science that can actually make a difference.
Chemicals like 1-(3-Chloropropyl)-4-Methylpiperazine never take a day off. In research, manufacturing, or teaching labs, safety steps shape every move. I remember one winter in a chemistry teaching lab, a misplaced bottle of solvent forced an evacuation—no one ever forgot that lesson about storage.
Most chemical accidents start with neglect or a shortcut. 1-(3-Chloropropyl)-4-Methylpiperazine carries risks typical of chlorinated organics—skin and eye damage, vapor inhalation risks, and environmental harm. Ignoring even one aspect of storage quickly turns a well-ordered work area into a hazard. OSHA figures show thousands of yearly incidents connected to improper chemical management. Tales of corroded shelving, melted gloves, and ruined experiments echo across research buildings everywhere.
Temperature ConsistencyRoom temperature generally works for most organic compounds, and this one fits the mold. Avoiding direct sunlight and heat sources goes a long way toward maintaining stability. I’ve seen how summer heat can warp bottles and pop caps, creating leaks that went unnoticed until the scent reached the hallway. Unless a container’s manufacturer urges refrigeration, consistent room temperature—typically in the 15-25°C range—protects your workspace and your team.
Sealed ContainersAir and moisture seep into poorly closed jars. Chlorine-containing compounds and piperazines can react or degrade, sometimes forming hydrochloric acid or messy byproducts. Only bottles rated for chemical storage—tightly capped and labeled—belong in your cabinet. I once inherited unlabeled, mismatched containers in an academic stockroom; each one demanded double-checking, extra PPE, and disposal. Nothing busts a budget like waste disposal for mystery chemicals.
Ventilation and PlacementStrong smells and vapors build up in stuffy rooms. Good storage means using flame-proof cabinets with vents in well-aired spaces. Make sure the chemical sits far from oxidizers, acids, or reducing agents. Mixing incompatible bottles on a single shelf just tempts fate. In my old lab, separate cabinets, clear hazard signs, and strict shelving rules stopped accidents before they started.
Containment for SpillsSpills ruin more than experiments. Simple trays or secondary containers hold leaks and prevent chemical creep across shelves. Whenever I spot cracked glassware or a sticky residue, I grab containment bins right away, then check the entire shelf for problems.
Old or faded labels create confusion. Use bold, legible chemical names, hazard pictograms, and dates on every bottle. Inventory checks turn up expired or suspect materials before they become a problem. The strongest labs I’ve worked in set up monthly walk-throughs, keeping their chemical stocks predictable and safe.
Safety grows from small habits repeated daily. No policy replaces vigilance or responsible handling. Most best practices grew out of near-misses and painful lessons. Encouraging all team members to speak up about storage issues saves time, money, and sometimes much more.
Every bottle on a shelf carries a story—of careful planning or a warning from the past. Storing 1-(3-Chloropropyl)-4-Methylpiperazine in cool, dry, labeled, and secured conditions makes scientific work safer, smarter, and more sustainable for everyone involved.
Working in and around chemical spaces, you start to notice how jargon sometimes hides the real risks. With 1-(3-chloropropyl)-4-methylpiperazine, some overlook the punch this compound can pack. It's tempting to treat it like just another tool on the lab shelf, but hands-on experience throws up warning signs pretty fast. Forget glossy safety sheets for a moment and remember what counts: skin, air, and surfaces.
One of the biggest mistakes you see in busy workplaces is handling stuff like this bare-handed. Skin contact raises concerns, and you don’t want it on your hands, arms, or any exposed skin. Reports from actual incidents—for example, staff handling piperazine compounds in cramped research labs—describe redness, itching, and sometimes a headache that lingers. This is a small-molecule organic with the potential to penetrate skin barriers, so standard nitrile gloves, not vinyl, become a ready solution. Always trade up for proper gloves and get comfortable with face shields during transfers.
Opening a fresh jar can release fumes that sneak up your nose. Odor might stay faint, but your respiratory tract pays the price. Chemicals containing chlorinated side groups cause coughing or even shortness of breath after repeated exposure, especially in closed-off spaces. Proper exhaust hoods and simple, well-fitted disposable masks make a difference right off the bat. From lived experience, stepping back just to get fresh air cuts down dizziness.
A lot of lab folk carry a sense of invincibility because acute reactions stay rare. But with repeated exposures, you stack up risk. There are published studies flagging possible toxicity of methylpiperazine derivatives, including cytotoxic effects in biological cells. What does that mean day-to-day? Don’t take chances on chronic headaches or the chance of altered blood counts. Label the container clearly and keep it away from food, water bottles, or personal items—it’s easy for accidents to happen after long shifts.
Small spills near the balance can create bigger headaches than many expect. This compound won’t simply evaporate safely into the air. Someone sweeping up powder without gloves risks direct contact, so use absorbent pads and follow with a proper chemical disposal, not a trip to the regular trash can. Training newcomers on the clean-up steps keeps everyone safer, and regular drills make each person’s habits sharper.
People learn best from clear, practiced habits and seeing supervisors set the example. Putting up reminders at the workbench beats relying on memory alone. Encourage open talk if someone notices a near-miss or feels unwell. Team members support each other better this way, and concerns get addressed before bigger problems arise.
Routine safety reviews identify shortcuts that may creep in, like skipping lab coats or forgetting to vent the workspace before starting. Rotating tasks keeps fatigue from becoming a hazard in itself; tired hands spill more than focused hands. Investing in fume hoods and powered air filters isn’t glamorous, but stinging eyes and persistent headaches cost a lot more down the line.
Respect for chemicals such as 1-(3-chloropropyl)-4-methylpiperazine grows out of daily discipline, not just theory. Everyone wins when risk stays in the spotlight—on the label, in the training, and in every step of the process.
People working in pharma labs, academic research, or specialty synthesis have probably crossed paths with some version of 1-(3-Chloropropyl)-4-methylpiperazine. This name might sound technical, but the day-to-day decisions about purity feel practical for researchers and product developers. Picking a lower purity could mean dealing with side-products and headaches in your experiments. On the flip side, a higher grade can hit your budget hard. Purity grades are not just about numbers — they set the tone for reliability, efficiency, and even safety of chemical reactions or downstream processing.
Most commercial suppliers list available grades above 97%. A small cluster offers 99% or above, mainly marketed to pharmaceutical developers or folks running critical syntheses. Higher purity can cut troubleshooting down the line, especially if chasing trace-level effects in your target molecules. Lower grades often show up as “technical”, and come with the classic catch: unknown impurities, unknown risks. Analytical chemists are the first to notice when an uncontrolled variable throws results off—which happens if a batch arrives with even half a percent of unknowns.
Major players, whether in North America, Europe, or Asia, list this compound in bulk quantities and research-scale vials. Large chemical marketplaces will turn up several sources selling 25g, 100g, up to multi-kilogram batches. Most of those ask for documentation and purpose of use. This step filters out non-research orders, and rightly so. In research settings, traceability adds a level of safety that never gets old for people running sensitive projects or compliance audits.
Several years ago, a project required the hydrogenation of a piperazine derivative. A single impurity in a batch wasted days—chromatography only showed its face after the reaction finished. That pain left a mark. Since then, sourcing always starts with a recent Certificate of Analysis, not an old one floating around the web. Colleagues echo this: a supplier’s willingness to talk details and show documentation matters more than flashy marketing or generic web listings.
People sometimes ignore purity until things go sideways. In regulated environments, one off-spec batch leads to lost time, paperwork, and (worst of all) failed compliance. GMP-certified, pharma, or “research only” labels mean different things to different folks. A guarantee looks better than a vague promise. Long-term, I’ve found that building relationships with reliable suppliers saves more time than chasing the cheapest price for each project. Laboratories tracking their long-term results see fewer negative surprises this way.
The market for 1-(3-Chloropropyl)-4-methylpiperazine keeps growing as people demand new molecules in diagnostics, pharmaceuticals, and specialty materials. Sourcing from suppliers who keep transparency front and center pays off. Reliable documentation and real technical support are worth their weight, especially when things go wrong. Researchers and companies can also push for strong batch-level transparency through their purchasing power.
Whether making a new active ingredient or running academic research, overlooking purity and traceability often leads to false economies. Genuine quality, while sometimes pricier at first glance, sidesteps extra analytical work and downstream disappointment. Making the right choice starts much earlier than the reaction flask: it starts with honest sourcing, reliable paperwork, and support that doesn’t dry up after the sale. This focus on substance—not just percentage points—defines whether science moves forward or grinds to a halt.
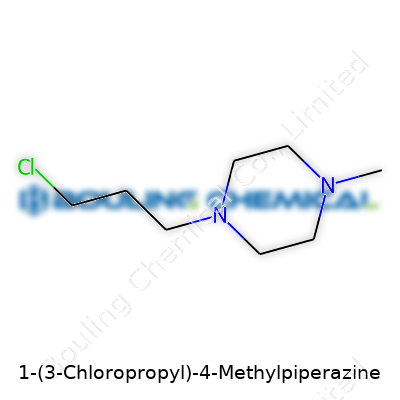
| Names | |
| Preferred IUPAC name | 1-(3-chloropropyl)-4-methylpiperazine |
| Other names |
1-(3-chloropropyl)-4-methylpiperazine 4-Methyl-1-(3-chloropropyl)piperazine 1-(gamma-Chloropropyl)-4-methylpiperazine 1-(3-Chloropropyl)-4-methyl-1,4-diazacyclohexane |
| Pronunciation | /ˈwʌn θriː-klɔːrəˈproʊpɪl fɔːr ˈmɛθɪl paɪˈpɛrəziːn/ |
| Identifiers | |
| CAS Number | [356570-52-8] |
| 3D model (JSmol) | `CCCClN1CCN(CC1)C` |
| Beilstein Reference | 1718734 |
| ChEBI | CHEBI:189384 |
| ChEMBL | CHEMBL266579 |
| ChemSpider | 156668 |
| DrugBank | DB08375 |
| ECHA InfoCard | 03a5e2f9-6691-49da-b623-8c04b51c1e7c |
| EC Number | 4334-06-3 |
| Gmelin Reference | Gmelin 84155 |
| KEGG | C18768 |
| MeSH | C033086 |
| PubChem CID | 141656 |
| RTECS number | GV9945000 |
| UNII | 1Y7A0S2B2S |
| UN number | UN3439 |
| Properties | |
| Chemical formula | C8H17ClN2 |
| Molar mass | 212.72 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Odorless |
| Density | 1.08 g/cm³ |
| Solubility in water | soluble |
| log P | 1.97 |
| Vapor pressure | 0.0415 mmHg at 25°C |
| Acidity (pKa) | pKa = 9.8 |
| Basicity (pKb) | pKb = 4.89 |
| Magnetic susceptibility (χ) | -77.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.508 |
| Viscosity | Viscosity: 3.06 cP (25°C) |
| Dipole moment | 3.85 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 403.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -144.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4947 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 95°C |
| Lethal dose or concentration | LD50 oral rat 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | Not Established |
| REL (Recommended) | REL: Not established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-(3-Bromopropyl)-4-methylpiperazine 1-(3-Chloropropyl)piperazine 1-(3-Chloropropyl)-4-ethylpiperazine 1-(3-Chloropropyl)-4-phenylpiperazine 1-(2-Chloroethyl)-4-methylpiperazine |