Chemical innovation never stands still. The story of 1-(2-Methoxyphenyl)Piperazine traces back to the late 20th century, as chemists set out to probe new heterocyclic structures for pharmaceutical development. Research ramped up during the 1980s, with European labs focused on arylpiperazine derivatives like this one, hoping to find affordable, versatile building blocks for emerging psychotropic therapies. Journals from that era highlight growing awareness around neuroreceptor ligands and their therapeutic relevance. This compound kept popping up in medicinal chemistry papers due to its link to serotonergic and dopaminergic systems, nudging scientists to explore its broader chemical and biological potential.
Today’s chemical supply catalogs often include 1-(2-Methoxyphenyl)Piperazine as a specialty intermediate. Manufacturers usually present it as either a crystalline solid or a colorless to light yellow oil, packed in airtight glass containers due to its sensitivity to air and light. What makes this structure stand out is the distinct placement of a methoxy group on the phenyl ring, which sets the backdrop for a diverse set of reactions. Chemists, especially those working with CNS-active agents or receptor-binding studies, keep reaching for this compound thanks to that unique scaffold.
You want reliability with any chemical, and 1-(2-Methoxyphenyl)Piperazine doesn’t disappoint. It typically melts between 72–76°C. The molecule weighs in at 206.28 g/mol, and the methoxy group brings an element of electron-donation, which shifts the reactivity compared to the unsubstituted variants. It dissolves well in polar organic solvents like ethanol, methanol, or dimethyl sulfoxide. Some batches appear slightly sticky at room temperature if the humidity rises—an annoying detail for those working without a fume hood. Chemical stability holds up under cool, dry conditions, but the compound gives off that sharp aromatic amine smell if mishandled. Simple tests, like TLC and HPLC, confirm that impurities or degradation products rarely slip past a careful eye.
Suppliers should display every batch’s purity profile, documented using GC-MS, NMR, or HPLC. Labels always show the exact concentration, batch number, synthesis date, and recommended storage temperature. Hazard symbols matter; most vials carry strict cautions: “For research use only,” and clear guidance on PPE—eye protection, gloves, and lab coats every time you open that bottle. Stringent compliance with local and global regulatory benchmarks (such as GHS and REACH for the EU) builds user trust and prevents legal headaches. The best suppliers also tack on up-to-date safety data sheets, making it easy for any lab to assess risks and plan controls.
Synthetic approaches have evolved as demand has shifted from bench to pilot scale. A classic route involves reacting 2-methoxyaniline with phosgene, forming the intermediate carbamoyl chloride, which then meets piperazine in a controlled condensation. Some chemists go for catalytic hydrogenation of 1-(2-methoxyphenyl)piperazin-2-one, bypassing the need for hazardous reagents and minimizing purification steps. Reactions usually run under anhydrous conditions using aprotic solvents like dichloromethane, and the yields improve dramatically by slowly adding reactants and monitoring temperature closely. Post-reaction workups use either solvent extraction or column chromatography to achieve the high purity demanded by research teams, and waste disposal calls for incineration or certified solvent reclamation, respecting environmental best practices.
1-(2-Methoxyphenyl)Piperazine lends itself to robust modification. The methoxy site acts as a springboard for demethylation, revealing the free phenol, which opens even more chemistry. Electrophilic substitution—such as nitration or halogenation—lets chemists tweak the electron density on the phenyl ring, tailoring pharmacological profiles. The piperazine ring often gets alkylated, acylated, or sulfonated, sometimes leading to improved passage through biological membranes. Medicinal chemists have found that the core structure responds well to coupling reactions like Suzuki or Heck, which expand chemical diversity for SAR studies. Each tweak can nudge potency, selectivity, or metabolic profile.
Labs and catalogs speak many chemical languages. You might see this compound listed as o-Methoxyphenylpiperazine, 2-Methoxy-1-piperazinylbenzene, or 1-(2-methoxyphenyl)piperazine hydrochloride (when stabilized as a salt). In some European pharmacology studies, researchers abbreviate it to oMeO-PP or MoPP. These different names make labeling checks crucial before any experiment, minimizing dangerous mix-ups between structurally similar piperazine derivatives.
This compound doesn’t reward carelessness. Handling always starts with a walk through the Safety Data Sheet—easy access protects everyone on the team. The powder can cause irritation to skin, eyes, or mucous membranes. It’s tempting to skimp on gloves or masks during a busy day, but even light exposure causes redness or headaches. I learned long ago to ventilate the hood well and double-bag any spills for prompt cleanup. Some countries classify arylpiperazines as controlled substances or precursors—so paperwork and inventory tracking matter. Training refreshers for all personnel, not just the new hires, keep labs in line with both ethics and law.
Medicinal chemistry treats 1-(2-Methoxyphenyl)Piperazine as a tool to construct library molecules, many of which look promising as receptor agonists or antagonists. Its framework pops up in compounds with antidepressant, anxiolytic, or antipsychotic actions. Outside pharmaceuticals, researchers turn to it to probe serotonin and dopamine pathways in neuroscience studies. Some materials scientists experiment with piperazine derivatives, aiming to develop novel polymers or supramolecular assemblies, though pharmacology remains the prime user base. Academic papers over the past decade keep citing the methoxy-variant for its decent balance of activity and selectivity, especially in serotonin receptor binding assays.
Universities and drug companies have both plunged into SAR analysis, building out analogues from this piperazine core. Studies often track how modifications shift receptor affinity, blood-brain barrier penetration, or metabolic stability. Several Japanese and European teams have published synthetic schemes yielding more selective ligands for the 5-HT1A and 5-HT2A receptors, two targets implicated in mood and perception. Screening algorithms often highlight the methoxy group as a sweet spot for modulating both on-target and off-target effects. I’ve watched collaborators gain traction using this intermediate, especially in iterative lead optimization, because it offers reliable reactivity and biological versatility.
Animal models and in vitro assays point out clear limits. The central nervous system shows the most sensitivity, with dose-dependent sedation or motor deficits in high concentrations. Chronic studies suggest some risk for mild hepatotoxicity, though metabolites clear efficiently in healthy systems. Cell-based assays find cytotoxicity only at concentrations that exceed normal research protocols by several orders of magnitude. Carcinogenicity or genotoxicity hasn’t appeared in published literature, but comprehensive longitudinal studies lag behind its clinical cousins. Lab policies should minimize aerosol formation and prevent accidental ingestion, and waste streams ought to run through monitored chemical disposal, given its bioactivity.
Looking ahead, interdisciplinary research holds promise for new applications. Cheminformatics platforms now screen thousands of piperazine-derived molecules, flagging o-methoxy variants as valuable scaffolds for CNS-targeted drug discovery. Grants increasingly support green chemistry for arylpiperazine synthesis, with teams replacing hazardous reagents with safer precursors and recyclable catalysts. Personalized medicine and receptor profiling could push chemists to revisit this compound, seeking higher selectivity or metabolic stability for next-generation treatments in psychiatry and neurology. My own interactions with early-career chemists show growing interest in this class, not just for drug design, but for fundamental studies on neurotransmitter pathways and molecular recognition. Safety, regulatory, and ethical diligence must continue to pace every step—so this molecule can keep delivering answers where research meets application.
1-(2-Methoxyphenyl)piperazine, often called oMeOPP or 2-MeOPP, shows up regularly in research articles and industrial registries. Chemists group it with the piperazine class. Structurally, it carries both a piperazine ring and a methoxy group attached to a phenyl ring. This design matters a lot in terms of how researchers use it and interpret its effects in various studies.
Many chemists pay close attention to this compound because it has potential effects on neurotransmitter systems, particularly serotonin and dopamine. I first heard about it from a graduate student who works on central nervous system projects. They look to compounds like 1-(2-Methoxyphenyl)piperazine as tools, attempting to map out brain receptor activity or to demonstrate how certain drugs might affect the mind. Rather than ending up in medications, structure-activity research with compounds like these guides safer drug development paths. For example, one study in Neuropharmacology used it to study serotonin receptor selectivity—valuable for understanding depression and anxiety treatments.
Pharmaceutical chemists often start with these molecules to build new candidates for medications. They layer extra parts onto the base structure or tweak functional groups, trying to reach something with a helpful effect and a better safety record. A good deal of foundational knowledge comes from early results with research chemicals like 1-(2-Methoxyphenyl)piperazine. These efforts, mapped out in patents and published work, feed into new drugs for mood disorders, pain, and beyond.
Over the past decade, the growth of online markets and gray-area head shops means some compounds, originally researched by scientists, have found other routes to the public. 1-(2-Methoxyphenyl)piperazine shows up occasionally in chemical catalogs or seized product analysis reports. Law enforcement and forensic labs have documented this trend, especially as people seek new substances not covered under classic drug laws. This kind of off-label use creates a headache for health officers and researchers alike, as safety testing falls far behind the pace of distribution. Reports from the European Monitoring Centre for Drugs and Drug Addiction indicate that various piperazines have been detected in counterfeit pills or as additives in party drugs. These products rarely get manufactured with quality controls, amplifying risks linked to purity and unknown interactions.
Researchers using 1-(2-Methoxyphenyl)piperazine follow strict guidelines to ensure safety and reliability. Laboratory protocols require careful measurement, and institutional review boards monitor potential harms and ethical risks. In contrast, products containing these compounds from unofficial sources might carry unknown or dangerous byproducts. Inconsistent supply chains leave people guessing about what’s really in a sample. Back in my university lab days, the chemical storeroom had strict sign-out rules for experimental agents like this—and for good reason. That level of caution keeps both people and data safe.
Clear, enforceable laws help limit unsupervised distribution of substances like 1-(2-Methoxyphenyl)piperazine to the public. Improved training for medical and law enforcement professionals can make identification swifter and responses more targeted. Funding surveys and analytical labs helps health authorities track change and respond faster when these molecules surface outside controlled settings. At the same time, continued basic research under regulated conditions broadens understanding of how these chemicals interact with brain and body. Each element supports the other: legal oversight protects communities; research helps inform those laws and keeps the field moving forward safely.
Curiosity about lesser-known chemicals often leads people down a rabbit hole of legal ambiguity. 1-(2-Methoxyphenyl)piperazine, known in some circles as oMPP, belongs to the piperazine class. It has popped up in various reports about research chemicals and substances that sit somewhere between useful research tools and party drugs, depending on who’s asking. The big question hopping around the internet: can someone pick up oMPP legally?
No blanket answer fits every country. In the United States, this compound doesn’t show up as a named controlled substance at the federal level. But that doesn’t make it an open invitation to order from any online source. Prosecutors sometimes use what’s called the Federal Analog Act. They try to build cases by arguing that a chemical, even if not listed by name, is essentially chemically and functionally similar to a banned drug—in this case, maybe a close cousin to mCPP or other piperazines listed in Schedule I. Some states will have their own laws that add to federal requirements and might list piperazines or specific analogs outright.
Europe brings its own mix. Some countries—like the United Kingdom—have taken a broad sweeper approach, putting entire families of substances under their Misuse of Drugs Act. Other places, like Germany, have their Narcotics Act that tends to update over time as new chemicals pop up in the market. Then there are those countries without a specific mention of oMPP and without blanket analog laws, making the legality a bit like legal Russian roulette: technically possible, but risky.
Chasing research chemicals for any use—scientific or otherwise—brings more than legal headaches. Purity and legitimacy sit on shaky ground when working with unregulated markets. Labs selling substances online often cross international borders, and that opens up packages to customs inspections or, worse, seizures. I remember talking with college friends many years back who ordered something similar, only to find nothing but a letter from customs minus their parcel.
Health plays an equally big role. There’s a clear lack of published research into long-term effects or safe dosing for many chemicals in the piperazine family. No guarantee exists of quality control, so anyone purchasing these substances might face real physical risks from contaminants or mislabeling. Illegal online sellers toss around lab terms like “for research only,” but that rarely equals actual scientific scrutiny.
If the demand for compounds like 1-(2-Methoxyphenyl)piperazine stems from gaps in treatment or curiosity about mind-altering substances, it’s a warning flag for better science and more public discussion. Public health agencies and scientists could do more work documenting the presence and effects of these compounds, giving clear information for clinicians and policymakers.
For those who work in chemistry or pharmacology, responsibility means staying up to date on the legal landscape and thinking ahead about unintended consequences. Laws might always lag behind fast-moving chemistry, but taking time to consider both safety and legality offers a steadier path.
Every country has its own approach, so someone thinking about purchasing any research chemical would be wise to check with local legal expertise instead of leaning on open forums or internet hearsay. Relying on major government agencies and academic resources beats out word-of-mouth every single time. Practicality wins out: staying on the right side of the law and health safeguards always makes more sense than chasing loopholes.
1-(2-Methoxyphenyl)piperazine, often called oMeOPP, belongs to the family of phenylpiperazines. Compounds in this group sometimes get explored in the development of pharmaceuticals, particularly for their effects on serotonin pathways in the brain. Despite its presence in some scientific studies, oMeOPP does not hold approval for any medical use. People might come across it in research settings or, less commonly, in recreational circles, sometimes misused for its psychoactive effects.
Having worked in a pharmacy, I’ve seen the way even lesser-known compounds can spark interest once word spreads about their subtle mind-altering properties. With oMeOPP, the body doesn’t always respond kindly. Most common are headaches, nausea, and a racing heart. Some folks describe tingling sensations in their hands and feet. This matches up with what pharmacologists observe: phenylpiperazines often cause lightheadedness and muscle tension, and in certain cases, tremors. None of it tends to feel pleasant.
On the mood side, users sometimes report anxiety, restlessness, or trouble sleeping. A study from the early 2000s highlighted similar reactions among healthy volunteers subjected to related piperazine compounds. They complained about jittery feelings and trouble concentrating. This didn’t surprise the researchers; tinkering with brain chemistry, especially serotonin, often kicks up unpredictable mental fog. The risk of feeling out of sorts seems real enough to deter casual experimentation.
Side effects never act in isolation. Increased heart rate and blood pressure can place older adults or those with heart issues at higher risk. Even young people, if using it alongside other stimulants or antidepressants, might stumble into trouble. There’s a real worry about serotonin syndrome—a condition caused by too much serotonin—when combining oMeOPP with other drugs that work on the same brain pathways. Symptoms can include confusion, muscle rigidity, and, in dangerous cases, seizures.
Not much is known about the long-term risks. No high-quality, peer-reviewed studies track individuals over time after oMeOPP use, but similar drugs have caused persistent anxiety and sleep troubles for weeks in some people. The drug’s unpredictable nature means that anyone thinking about experimenting could end up feeling off-balance or worse for a while.
Molecules like oMeOPP look simple under a microscope, but once they interact with the body, things seldom stay simple. There isn’t a safety net for people who try these compounds outside the lab. Medical supervision and proper dosing protocols act as guardrails for approved medications. With chemicals like this, those safety rails simply aren’t there. Stories from online forums hint at people mixing oMeOPP with other substances without understanding the risks, which magnifies the potential for harm. Reliable, science-backed information often lags behind the curiosity of those eager to experiment.
Protecting people from harm begins with education. Health professionals need resources to explain the dangers of experimental drugs, especially to younger populations. Outreach in schools, clinics, and through digital platforms can help cut down curiosity-driven use. When somebody ends up in the emergency room after trying a compound like oMeOPP, their stories can serve as a warning. Policymakers and health agencies must support research, keep the public updated, and stay ahead of the changing landscape of new compounds hitting the market.
In the end, best results usually follow a measured, skeptical approach to anything that tinkers with brain chemistry. Science and personal health both benefit from patience and clear-headedness.
Every time I’ve worked in a lab, the way we store chemicals can mean the difference between a safe work day and a real mess, or even a disaster. 1-(2-Methoxyphenyl)piperazine falls into a group of organic chemicals that can react, degrade, or spoil if left in the wrong corner or next to the wrong bottle. The basics seem obvious—don’t just toss it on any shelf. There’s a reason lab managers insist on rules.
Based on what experts and chemical safety data tell us, 1-(2-Methoxyphenyl)piperazine should stay in a cool, dry spot, far away from direct sunlight and sources of heat. Sunlight breaks molecules apart and heat speeds up chemical reactions. Over time, even a small change in temperature or humidity can turn what should be a reliable chemical into something unpredictable. In my early years handling double-wrapped serotonin agonists, occasional vials would yellow or clump just from skipped protocols.
Moisture messes with purity. Humidity can creep into containers that aren’t sealed tightly, especially if someone rushes and doesn’t replace a cap right away. Contaminated chemicals don’t just throw off experiments; they can put people in danger if unwanted reactions start. A tight seal using a screw-cap or crimped vial limits air and water exposure. Whenever I see vials in exposed glass jars or soft plastic, alarm bells go off. I remember the time a colleague lost a week’s work by storing sensitive compounds next to the water bath—simple steps matter.
Organization stops accidents before they happen. Any person pulling from shelves should spot clear labels, hazard warnings, and production dates right up front. Chemicals like this belong apart from strong acids, bases, and especially oxidizing agents. Mixing incompatible groups in one cabinet invites chemical fires or toxic vapors. I’ve seen near-misses from rushing during inventory checks. Separating each class makes emergencies less likely and clean-up practical if a spill or break does happen.
Stable room temperature works for some chemicals, but it doesn’t mean every lab bench fits the bill. Temperature swings—like the kind everyone sees near windows or air vents—can wreck stability. Temperature logs or alarms help, especially for chemicals that start breaking down above certain points. I started trusting digital loggers after a storm knocked out power for hours. Without records, there would have been doubts about every batch stored in that room. Better to catch a problem with a reading and discard a single vial than lose confidence in everything kept there.
Expired or degraded chemicals shouldn’t hide in the back, where people forget until it’s too late. Safe disposal protects more than just individual users. Waste management policies make a difference in every lab or company site. Responsible labs document stock and removal, and I’ve found that tracking lot numbers and keeping updated logs pays off in time saved and headaches avoided.
The baseline for good storage starts long before anything reaches a shelf: checking up-to-date safety datasheets, investing in quality containers, and training every new hire on why shortcuts cost more in the end. Secure storage, clear labeling, regular audits, and strong oversight tie all this together. Drawing from years in research and teaching, I’ve seen that the labs with a culture of care—where everyone looks out for red flags—end up with better science and safer people. Simple habits built into daily routines make the difference.
Discussions about 1-(2-Methoxyphenyl)piperazine, often called o-MeOPP or ortho-methoxyphenylpiperazine, walk a risky line between scientific information and underground knowledge. There’s no FDA guidance on safe dosage, simply because this compound never earned legitimate medical status. Chemists created it as a research compound, with some users calling it a recreational “piperazine.” Most people first heard about it from online forums rather than any doctor’s office. That leaves many confused about proper dosages and the possible hazards they’re signing up for.
Clinical trials on o-MeOPP are hard to come by. Instead, experienced users cobble together reports from websites, usually quoting doses in ranges from 20 mg to 200 mg, depending on desired effects and previous experience. These numbers come straight from anecdotal reports, not peer-reviewed studies. That’s a serious gap, and it’s dangerous to ignore. Nobody can say how the average person metabolizes this compound or what kind of long-term damage might crop up. The human body isn’t a chemistry set, so what looks okay on a sheet of paper can turn ugly in real life.
Among the piperazine family, several molecules landed on banned lists worldwide after people experienced fatal reactions. Compounds like BZP and mCPP seemed mild at first, only for users to later report panic attacks, dangerously high blood pressure, and hospitalizations. Authorities in some countries clamped down, making these drugs illegal to possess or sell. 1-(2-Methoxyphenyl)piperazine hasn’t had as much study, but risks seem similar. Users sometimes report nausea, sweating, confusion, and racing heartbeats. No one has published a safe, controlled study about potential overdose or cumulative damage.
Online communities offer harm-reduction advice, but users run on the hope that their own biology won’t betray them. Dosage charts on forums or with pill-testing kits may work for someone else, but two people’s livers and brains never process drugs the same way. Add in unregulated pill composition or contaminated powder, and that “safe” dose can become anything but. When friends try to help friends dose up, they rely on guesswork instead of science. That method can backfire fast, leading to tragic emergencies nobody expected.
Most countries respond to unknown research chemicals by banning them outright, but the black market rarely dries up that easily. Real solutions start with honest information—not scare tactics, but cold facts and firsthand accounts. Schools, clinics, and community centers could do more to reach young people where they are, with current information and help lines staffed by people who understand street drug trends. Testing services that check pills for dangerous additives can save lives, although those run into funding and legal barriers in some places.
Public health requires more than regulation; it also needs respect for people’s reality. Users deserve factual advice, open access to testing kits, and easy- to- find medical support when things go wrong. If governments and health systems accept the need for honest dialogue—guided by teachers and doctors with real street experience—fewer people will get hurt by compounds like 1-(2-Methoxyphenyl)piperazine. Until science catches up, the safest dosage is zero.
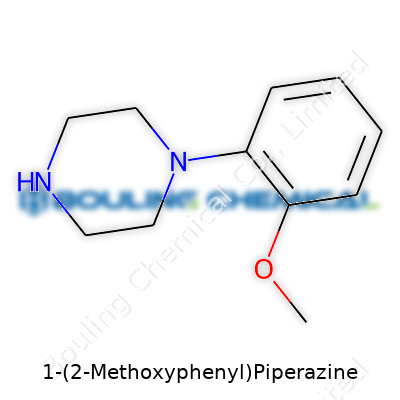

| Names | |
| Preferred IUPAC name | 1-(2-Methoxyphenyl)piperazine |
| Other names |
o-Methoxyphenylpiperazine 2-Methoxy-1-(piperazin-1-yl)benzene 2-MeO-PP 1-(2-Methoxyphenyl)piperazine |
| Pronunciation | /ˈwʌnˈtuː mɛˈθɒksoʊˈfiːniːl paɪˈpɛrəˌziːn/ |
| Identifiers | |
| CAS Number | [23639-24-1] |
| Beilstein Reference | 120856 |
| ChEBI | CHEBI:131278 |
| ChEMBL | CHEMBL14090 |
| ChemSpider | 145977 |
| DrugBank | DB00839 |
| ECHA InfoCard | 100_000_085 |
| EC Number | 627-229-4 |
| Gmelin Reference | 71668 |
| KEGG | C11265 |
| MeSH | D08.811.939.880.500.400 |
| PubChem CID | 71335 |
| RTECS number | TK3156000 |
| UNII | W6C8EE8G6O |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID4020731 |
| Properties | |
| Chemical formula | C11H16N2O |
| Molar mass | 222.30 g/mol |
| Appearance | Light yellow liquid |
| Odor | amine-like |
| Density | 1.1 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 1.62 |
| Vapor pressure | 1.44E-3 mmHg at 25°C |
| Acidity (pKa) | 7.45 |
| Basicity (pKb) | 7.84 |
| Magnetic susceptibility (χ) | -62.82·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.590 |
| Viscosity | Viscosity: 0.964 mm²/s at 25 °C |
| Dipole moment | 3.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 296.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4054 kJ/mol |
| Pharmacology | |
| ATC code | N06AX11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-🌟 |
| Flash point | 107°C |
| Lethal dose or concentration | LD50 (oral, rat): 180 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 210 mg/kg |
| NIOSH | MW8560000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1-(2-Methoxyphenyl)piperazine is not specifically established by OSHA or ACGIH. |
| REL (Recommended) | 0.39 mg/m³ |
| IDLH (Immediate danger) | NIOSH: Not listed |
| Related compounds | |
| Related compounds |
1-(2-Ethoxyphenyl)piperazine 1-(2,3-Dimethoxyphenyl)piperazine 1-(3-Methoxyphenyl)piperazine 1-(4-Methoxyphenyl)piperazine 1-(2-Chlorophenyl)piperazine 1-Benzylpiperazine 1-Phenylpiperazine |