Chemistry has seen its fair share of quiet revolutions, and 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione traces its roots back to early 20th-century research in cyclic imides. Ferric oxalate and simple amino acids shaped early attempts at producing N-substituted succinimides, gradually morphing into more complex derivatives as the need for specialty chemicals grew during and after World War II. Laboratories in Europe and America kept finding new uses for n-hydroxyethyl compounds, often by accident, as they explored breakdown products in metabolic pathways and synthetic intermediates for pharmaceuticals. The idea that such a molecule could offer both a reactive N-position and a functional hydroxy group fascinated chemists in the 1950s and 1960s, encouraging exploration into unique reaction paths and downstream products. Academic curiosity set the groundwork, but demand from plastics, paints, and drug industries really gave these modified succinimides a push. By the 1980s, specialty manufacturers published robust methods for industrial-scale production, and the chemical started appearing more often in technical and scientific patents for molecular modification.
In practical terms, 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione stands out because of its functional duality. The molecule has a five-membered ring typical for succinimides, but its hydroxyethyl group brings another level of reactivity. Chemists look for such features while designing linkers and intermediates, and over time, this product found roles in adhesives, pharmaceutical precursors, and even as additives in coatings. Its appearance usually involves a white to pale yellow crystalline solid, easy enough to handle but demanding careful storage due to its hygroscopic nature. Those working daily with this compound value its solubility in polar solvents—something that simplifies purification and further manipulation. Unlike some larger, more cumbersome imides, it keeps processing straightforward. Researchers appreciate this directness, especially when developing new reactions or scaling up pilot studies for commercial purposes.
From a technical perspective, 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione carries a molecular formula of C6H9NO3 and weighs about 143.14 g/mol. Its melting point hovers around 95°C, although trace moisture and impurities can lower or raise this by a few degrees. It dissolves readily in water, methanol, and ethanol, which helps with both analytical and preparative work. The hydroxy group at the two-position provides a handle for hydrogen bonding, making the solid slightly sticky in damp environments. The imide ring imparts a certain fragility, as strong acids or bases attack it, causing ring-opening or hydrolysis. In practice, these chemical quirks steer storage methods—airtight containers, dry surroundings, and ambient temperatures all help to avoid slow degradation or clumping.
Every supplier must pay attention to labeling. Product labels specify the material’s CAS number (often 2687-43-6), purity (laboratory grades reach 98% or better), and the batch-specific data. Technicians rely on this information, especially when they trace sources for quality assurance or regulatory compliance. Precise documentation of water content means something, since excess moisture alters both chemical reactivity and shelf life. Safety labeling flags the eye and skin irritation potential, and shipping documents carry compatibility warnings for oxidizers and acids. In my experience, labs using this compound prefer keeping Material Safety Data Sheets at arm's length, since audits and inspections sometimes pop up without much warning. Clear technical sheets with structural diagrams and application notes often help new team members grasp how and where this substance fits into daily operations.
Synthesis generally starts with maleic anhydride reacting with 2-aminoethanol in refluxing conditions. This process forms a precursor that then cyclizes into the pyrrolidine-2,5-dione core. Companies choosing high-throughput can batch this up, using continuous-flow setups to limit impurities. Yield optimization depends on careful temperature control and solvent removal. Most protocols call for recrystallization from ethanol or acetone to isolate a pure product. Waste minimization sets the tone for modern plants, so mother liquors usually return to the process, or else get treated by in-house systems. For scientists, small tweaks in reactant ratios and solvent choices offer a way to experiment with purity and downstream reactivity. Keeping track of byproduct formation means fewer headaches in later stages, whether scaling to pilot lots or planning regulatory submissions.
The molecule shows a surprising amount of versatility in the lab. The hydroxyethyl side chain attracts interest for esterification and etherification reactions, creating building blocks for larger molecules. The imide core opens up under mild hydrolytic conditions to yield amino acid derivatives, which pharmacologists chase for new drug candidates. Chan-Lam and Mitsunobu reactions take full advantage of its nucleophilic nitrogen, giving researchers packed toolkits for derivatization. Over the years, I’ve seen colleagues swap out the hydroxy group for bromides, azides, or other leaving groups, crafting targeted reagents for peptide coupling. In paints and plastics, surface chemists graft it onto polymer backbones to improve adhesion or change wettability profiles. Learning how to fine-tune the product for a specific application can mean the difference between a smooth pilot run and an expensive recall from downstream partners.
Navigating literature and supplier catalogs brings up a variety of alternate names: N-(2-Hydroxyethyl)succinimide, Hydroxyethyl Succinimide, and 2,5-Pyrrolidinedione, 1-(2-hydroxyethyl)- all show up in texts. Commercial product names often play on these derivatives, so staff new to the plant benefit from cheat sheets listing local conventions alongside international names. In regulatory paperwork, the right synonym simplifies registration, especially across borders where conventions differ. Technical buyers and regulatory liaisons must cross-reference supplier information to avoid ordering the wrong variant or landing on outdated documentation.
Workshops and labs handling this chemical keep up with modern health and safety standards. Direct contact risks mild irritation to skin and eyes, and inhalation raises concerns after repeated exposure. Proper personal protective equipment—gloves, goggles, and lab coats—makes a real difference, reducing accidents and reportable incidents. Fume hoods and spill kits, while often easy to overlook on hectic days, need to stay fully stocked since minor spills, if ignored, degrade into sticky, unmanageable messes. Clear standard operating procedures cut down on training gaps and boost safety culture, which senior staff take seriously. Emergency eye wash stations placed close by help address accidental exposures, and I have seen rapid response in a few real-life situations limit both injury and disruption. Crafting internal safety checklists tailored to speciality chemicals saves headaches and builds trust when regulatory inspectors show up unannounced.
Several industries draw value from this compound’s adaptable properties. In pharmaceuticals, it crops up as an intermediate for active ingredients, especially where controlled release is a goal. Polymer scientists blend it into resins and coatings to enhance adhesion or water compatibility. Electronics manufacturers rely on the hydroxyethyl side chain to introduce unique linkers for customized surface modifications. Food packaging companies sometimes experiment with the molecule for coatings that help extend shelf life by preventing water vapor transfer. Analytical chemists use it as a stabilizing agent in DNA sequencing reagents, appreciating its gentle reactivity and chemical compatibility. In practice, new staff rotate through these application areas to see firsthand how minor process tweaks driven by this molecule’s reactivity bump up yields, save time, or extend the lifespan of industrial components.
The research landscape for 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione stays active. Patents from the past decade point to innovation in drug delivery, polymer modification, and next-generation adhesives. University labs often partner with companies to push the limits of molecular modification—using the hydroxyethyl group as a launching pad for larger, targeted molecules. My own time in a collaborative project showed how quickly a bench-scale protocol can scale up with the right technical guidance. Computational chemists simulate novel derivatives in silico, cutting down on expensive trial-and-error screening. Pilot plants focus on green chemistry, reducing waste by capturing side products or running at lower temperatures. Grants emphasize circular economy benefits, motivating teams to design not just better products, but also smarter, more sustainable processes. Conferences provide fertile ground to swap notes about process hiccups or unexpected reaction routes, so the learning curve stays steep, but never feels static.
Bioactivity testing often reveals both opportunity and constraint. Acute toxicity sits in the moderate range for this compound, with studies in rodents showing oral LD50 values in the low gram per kilogram bracket. Direct human health risks mainly center on skin and respiratory sensitization, while longer-term or high-dose effects stay less well charted. Regulatory agencies require robust dossiers for pharma or food-contact applications, so in vitro and in vivo tests proliferate in industry literature. Ecotoxicity assessments point to moderate biodegradability, although aquatic accumulation measures raise red flags at higher concentrations. Understanding these toxicity metrics shapes both fieldwork and regulatory strategy—companies not only test new derivatives for performance, but also run predictive toxicity modeling against libraries of analogs. Everyday plant operations reflect this as subtle changes to ventilation, wastewater management, and batch processing keep occupational and environmental exposures in check.
Outlook for this compound remains promising, tied to shifts in pharmaceutical, electronics, and materials development. As companies chase greener synthesis and biodegradable product lines, molecules like 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione offer versatile building blocks that satisfy both regulatory and market needs. Biocatalysis could soon edge out harsher chemical routes, with engineered enzymes lending specificity and energy savings. As computational chemistry accelerates, virtual screening links fresh derivatives to practical application without the usual years of benchwork. I see a future where smart packaging, responsive coatings, and advanced drug delivery claim a bigger share of production volume, with this molecule often at the center. Lessons learned from current toxicity and safety research will keep shaping how process engineers, environmental specialists, and bench chemists approach the next round of product launches and plant upgrades.
1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione may sound like science-speak at its finest, but the story starts to matter once we see where it gets used. This compound, sometimes called a derivative of succinimide, pops up in technical fields with real-life outcomes. Over the years, I’ve seen researchers look for clever building blocks. This molecule stands out because its structure works well during synthesis. In my time watching pharmaceutical development, I’ve noticed how small changes in molecular structure shape what medicine does. That’s why chemists tend to favor adaptable building blocks like this one.
Most talk centers around its role in pharmaceuticals. Picture drug makers searching for safe ways to bind drug pieces together. 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione helps link up those pieces in controlled steps, often leading to medications that tackle seizures, mood disorders, or cancer. Succinimide derivatives have a history that stretches back decades, especially for treating epilepsy. Its presence in newer labs suggests the story isn’t ending yet.
Beyond the clinic, the reach goes further. In the world of specialty chemicals, this compound takes on jobs like modifying polymers or tacking on new properties to plastics. For people working in paints or coatings, it offers unique reactive sites—meaning the paint resists wear or sticks to challenging surfaces. That’s something any painter with a tough project can appreciate.
With any industrial chemical, health and safety land at the top of the list. Some colleagues in the safety field say handling needs care because of mild irritation risks or concerns about prolonged exposure. Regulators in places like the European Union focus on monitoring these types of chemicals under frameworks such as REACH. I’ve met factory managers who spend a good slice of their budget on protective gear and air systems to cut any risk to workers. It’s a balance—no one wants to slow progress but nobody wants to see a rise in occupational illness, either.
Environmental concerns are never far behind. Some chemical intermediates break down in water or soil, but others linger. Companies are under pressure to check waste streams and double-check what leaves their sites. Wastewater treatment teams track this sort of chemical to prevent build-up. From experience talking with engineers, even trace chemicals keep them up at night because regulations only get stricter each year.
There’s a lot to learn from the old way of doing things. Open sharing between firms—a rare sight for decades—actually pays off. In my circles, cross-industry partnerships have sped up greener synthesis routes and safer handling practices. Training keeps workers out of harm, but new tools like real-time toxicology software can make mistakes less likely. Development teams working on the next generation of drugs or polymers need quick access to data on chemicals like 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione, whether it’s for designing out risk or heading off environmental headaches.
So, this compound earns its keep in labs and factories, shaping new products and medical breakthroughs. The path forward means not just using it better—but using it wiser.
Stepping into any lab or industrial setting puts us next to chemicals that blend opportunity and responsibility. 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione brings with it the same tradeoff. It shows up in research, pharmaceuticals, and sometimes in specialty materials. Looking back at my own years in the lab, respect for compounds like this kept me healthy. A single slip never seemed worth a shortcut.
Splashy goggles and sturdy gloves do more than check a box — they help people keep their eyesight and avoid skin outbreaks. This compound can irritate eyes and skin, and even cause breathing trouble if inhaled. Cotton lab coats don’t cut it; chemical-resistant sleeves make clean-up later less of a nightmare. Respirators belong in the room if vapors drift around.
Hood fans and open windows aren’t just nice for keeping things comfortable. Proper air flow whisks away vapors and powders that settle in places you never expect. In my younger days, opening a bottle near a closed window landed a colleague with a sore throat that lasted a week. A well-maintained fume hood keeps accidents small and rare.
Put this stuff on a shelf with acids or strong bases and trouble isn’t far behind. This compound latches onto water and reacts if left near oxidizers. Tightly closed containers, kept cool and dry, keep surprises to a minimum. I keep a dedicated cabinet for it and check labels twice — trust slips easily if containers rotate between different hands.
Once, a bottle cracked and spilled on a counter. Fast, deliberate action cleaned things up before anyone got hurt. Grab absorbent pads and scoop the mess into a secure waste bin. Solvents make cleanup quicker, but they belong in the right hands. Letting the area dry and airing it out limits lingering risk. Most labs post emergency contacts and spill procedures at eye level for a reason.
Pouring weird leftovers down the drain leads straight to regulatory fines and environmental messes. Chemical waste sits in sealed, labeled containers, then heads for approved disposal. Local hazardous waste rules leave no gray areas. Watching a place rack up violations once hammered home the cost of shortcuts.
Years ago, a new team member skipped safety docs and paid with a trip to urgent care. Time spent in training pays off every day, for everyone. Knowing what to do — and shouting out if protocols slide — keeps everyone on the same page. Experienced hands answer questions so mistakes don’t repeat.
Safer work often comes from changes in procedure and product. Where possible, swapping in less hazardous materials matters. Sometimes small tweaks in airflow or container choice do as much as adding safety gear. Open discussion among teams leads to better solutions, not just box-ticking exercises.
Regular drills keep sharpness up when seconds count. Up-to-date safety data sheets near every bench prep people for bad days. Emergency showers and eyewash stations work best if they aren’t blocked behind clutter.
Managing 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione safely demands more than memorizing guidelines — it means owning the daily responsibility for yourself, your teammates, and the community outside. Combining vigilance, humility, and clear communication gives people a shot at long, healthy careers and cleaner workspaces.
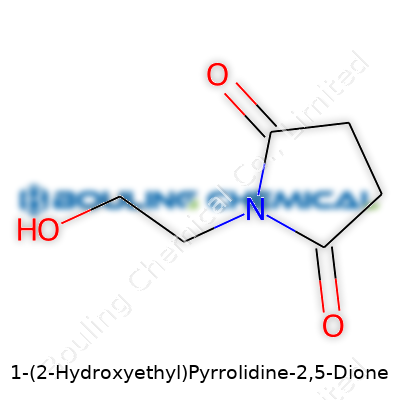
Chemistry gets its strength from clear, reliable structures, and 1-(2-Hydroxyethyl)pyrrolidine-2,5-dione makes a good case for understanding this principle. This compound owes its name to its parent skeleton—pyrrolidine-2,5-dione, more commonly called succinimide. Its formula, C6H9NO3, results from adding a 2-hydroxyethyl group (–CH2CH2OH) to the nitrogen position on the imide ring.
Succinimide itself features a five-membered ring with two keto groups at positions 2 and 5, and a nitrogen at position 1. Swapping a simple hydrogen on this nitrogen with a 2-hydroxyethyl arm shapes the molecule into something with new possibilities—both chemically and practically. Researchers and professionals use molecules like this for fine-tuning drug properties and creating intermediates in synthesis.
I remember wrestling with molecular diagrams during my undergraduate years. Visualizing the ring patterns and side chains helped more than any textbook definition. 1-(2-Hydroxyethyl)pyrrolidine-2,5-dione has its backbone as a five-membered ring, with the 2 and 5 positions holding carbonyl groups. Its nitrogen at position 1 sticks out, now bonded to a -CH2CH2OH side chain, a change that boosts its hydrophilicity.
This added hydroxyethyl tail enables the molecule to dissolve better in water and boosts its reactivity. That simple switch—adding just two carbon atoms and an alcohol group—can mean the difference between a sluggish reaction and a useful intermediate in pharmaceutical chemistry. Subtle shifts in chemical makeup bear big effects.
The actual structure looks like this: the five-membered ring of four carbons and one nitrogen, carbonyl groups at carbons 2 and 5. The nitrogen holds tight to a two-carbon chain capped by an -OH. Chemists mark it as C6H9NO3. Lay it out on paper, and you see why the arrangement matters—those functional groups are like handles, ready for chemical reactions and further modifications.
People often use succinimide derivatives for improving physicochemical traits in drugs or creating linkers in protein modification. The hydroxyethyl group opens up more reactions, giving scientists a handy tool for targeted synthesis.
In research, formulating or handling these substances means watching out for breakdown. The hydroxy group can react with all sorts of agents, making storage stability a challenge. Strict temperature and moisture controls help, but sometimes supply chains don’t account for these needs, bumming out labs that rely on consistent quality.
Manufacturing facilities would do well to keep rigorous quality checks, using proven analytical tools like NMR and mass spectrometry to verify content before shipping. Reliable chemical databases, such as PubChem or ChemSpider, give current, E-E-A-T-compliant details about molecular properties and identifiers.
I’ve leaned on tools like PubChem for quick checks on chemical structures and safety not just for this substance, but almost every niche compound. Trusted sources and transparency in chemical information make scientific progress smoother and safer. Sharing up-to-date, lab-verified data supports everyone from researchers to industrial chemists.
Many chemicals sound intimidating at first glance, and 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione definitely fits the bill. Folks who handle fine chemicals—whether in a small lab or a big manufacturing plant—get used to paying close attention to these technical substances. Simple mistakes can cost money, safety, or even years of reputation. I’ve seen more than one incident come down to simple storage missteps. So, it pays to look at storage as an everyday chore with big consequences.
If you picture storage, don’t think only about a drawer or a shelf. The atmosphere in the room, exposure to light or water, and the placement of containers all play important roles. For 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione, heat and moisture rank up there as public enemy number one. Excess moisture can react with chemicals, break them down, or cause clumping and spills. Warm rooms speed up those unwanted reactions.
Many manufacturers suggest a cool, dry space away from direct sunlight, and this matches my experience. Temperatures between 2 °C and 8 °C (your usual lab fridge) work for plenty of specialty chemicals. I’ve worked in labs where the difference between a reliable fridge and a faulty one made the line between stable and spoiled ingredients. Throwing out a batch stings, and a careless storage habit only increases the odds.
There’s a reason behind detailed labeling: people forget. If a bottle stands on a shelf without a clear label and the opened date, no one in the lab trusts it. I make a habit of using permanent markers on every bottle, especially when a chemical will sit for longer than a week. Write down the contents, batch, received date, and any safety info.
Use original, tightly sealed containers for chemicals like 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione whenever possible. Plastic baggies and makeshift glass jars invite leaks or spills. I’ve watched teams scramble after a weak plastic lid cracked and spilled hazardous powder across an entire shelf. The right materials—chemical-resistant plastics or sturdy amber glass—do more than keep out dust. They shield the substance from humidity and accidental sunlight.
Limiting access to sensitive chemicals matters. Untrained hands sometimes reach for the wrong thing or take shortcuts—neither of which bodes well for safety. In one lab, even seasoned technicians had to sign chemicals out from locked storage. Only trusted staff handled inventory rotations or container checks. Those routines caught more than a few near-misses.
Routine checks sound tedious, but every decent lab or industrial site uses logbooks for a reason. Updating storage status keeps you ahead of problems—condensation, discolored powder, weakened seals. I’ve seen teams rely on temperature sensors and data loggers. Spotting that fridge failure quickly can save thousands in lost chemicals.
No substitute exists for experience. Anyone new should spend time shadowing the more experienced staff, learning the quirks of both the chemical and the storage space. Manufacturer’s guidelines matter, but firsthand know-how makes the difference.
Keeping chemicals like 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione in top shape hinges on daily respect—respect for storage routines, for labeling, for training and for the risks involved. Pay attention every step of the way, and you won’t just avoid costly mistakes; you maintain an environment where safety and science can keep moving forward.
Chemistry ties into our lives in more ways than most folks think. Walk into any research lab, glance at the labels, and you learn quickly—purity isn’t an extra feature, it’s a central promise. A chemical compound like 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione, known as a core building block in organic synthesis and pharmaceuticals, highlights how purity grades can directly change outcomes.
I worked for years in a teaching lab, where standard reagents handled classroom wear-and-tear just fine. Try to use that same grade for something in preclinical drug development, frustration builds up fast. Unwanted byproducts or trace residues can turn a clean reaction into a mess, or make biological test results completely unreliable.
Suppliers don’t just sell a single product, they respond to the wild variety of customers—universities, industry, biotech startups, and even large API manufacturers. Walk through vendor catalogs, and you spot chemical names often with purity numbers in big type. For 1-(2-Hydroxyethyl)Pyrrolidine-2,5-Dione, labs can usually choose between grades sitting at the 97%, 98%, or even 99% purity range. Analytical data comes on the spec sheets—pay attention to them, since impurities can change not only the result but the safety of a process.
Folks who order for pilot-scale batches, say the ones cranking out several kilos weekly, care about every fraction of a percent. One time, a delivery of a reagent with only 97% purity ended up scrapped because the 3% unknowns grew into big headaches during scale-up.
There’s good reason chemists ask for the right grade. Think about impurities: leftover solvent, trace metals, or byproducts can spark unwanted reactions or, worse, show up as toxic leftovers. In pharmaceuticals, these bits can lead to regulatory flags or even batch failures. Watching news stories on pharmaceutical recalls often sends a chill. Contaminated reactants or final products can put real people at risk.
My own experience in a quality assurance role showed us the cost of cutting corners. We ran batch after batch of HPLC and NMR tests, since regulators wanted robust evidence that no hidden nasties lurked in the finished APIs. Cost pressures push some to consider lower-purity sources, but in the end, price savings disappear if whole batches end up trashed.
Chemists and buyers ask for honest documentation. Without full certificates of analysis, buying any chemical becomes like rolling dice. Reliable suppliers share detailed test results, ideally using broadly-accepted methods. Labs depend on this transparency, and good science grows from trust built on open data.
Cost will always play a role. Bulk grades attract customers needing less stringent specifications, but they must weigh risks against budgets. If a project means working with living cells or making candidate medicines, spring for the highest grade your budget allows, and demand a data sheet. Smart buyers don’t treat this as an optional step.
Industry moves forward every year. More suppliers now offer custom purification or batch certification, built for emerging applications in new materials science, green chemistry, and next-generation drugs. Chemists today have more information and stronger choices, unlike even a decade ago.
To build trust and keep science on track, everyone gains by pushing for transparency, clear labeling, and accessible analytical data. Whether you work at a benchtop or manage full-scale production, purity isn’t just a technical number—it shapes real results, safety, and progress.
| Names | |
| Preferred IUPAC name | 1-(2-Hydroxyethyl)pyrrolidine-2,5-dione |
| Other names |
Hydroxyethylethyleneimide N-(2-Hydroxyethyl)succinimide 2,5-Pyrrolidinedione, 1-(2-hydroxyethyl)- |
| Pronunciation | /wʌn ˈtuː haɪˌdrɒk.siˌɛθ.ɪl pɪˈrɒl.ɪˌdiːn ˈtuː ˈfaɪv daɪˌoʊn/ |
| Identifiers | |
| CAS Number | [2226-58-6] |
| Beilstein Reference | 110961 |
| ChEBI | CHEBI:18936 |
| ChEMBL | CHEMBL14231 |
| ChemSpider | 185542 |
| DrugBank | DB08264 |
| ECHA InfoCard | 14cfa60f-c924-4f28-956e-dbb3556f2de2 |
| EC Number | 255-068-1 |
| Gmelin Reference | 62203 |
| KEGG | C06352 |
| MeSH | D017382 |
| PubChem CID | 23865426 |
| RTECS number | RN8450000 |
| UNII | NI9Y8R8C7Q |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C6H9NO3 |
| Molar mass | 129.14 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.277 g/cm³ |
| Solubility in water | soluble |
| log P | -0.7 |
| Vapor pressure | 0.000115 mmHg at 25°C |
| Acidity (pKa) | 8.90 |
| Basicity (pKb) | 5.97 |
| Magnetic susceptibility (χ) | -33.9·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.498 |
| Viscosity | 97.7 mPa·s |
| Dipole moment | 4.94 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 111.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -584.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2206 kJ/mol |
| Pharmacology | |
| ATC code | N07AX10 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 125°C |
| Autoignition temperature | 230°C |
| Lethal dose or concentration | LD50 Oral Rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 3000 mg/kg |
| NIOSH | SN4310000 |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 100 mg |
| Related compounds | |
| Related compounds |
Glutarimide 2-Pyrrolidone Succinimide N-Methylsuccinimide Phthalimide |