Chemists first took a serious look at piperazine derivatives in the mid-twentieth century, searching for new structures that could anchor the next generation of medicines or intermediates. 1-(2-Furoyl)Piperazine didn’t just pop onto the scene out of nowhere—progress grew out of growing curiosity about heterocyclic compounds and their potential roles in many pharmaceutical and agricultural settings. As research labs branched out from simple piperazine rings, adding new functional groups and heteroatoms, scientists uncovered a range of molecules showing bioactivity and industrial value. The fusion of a furoyl group with piperazine marked an inflection point in attempts to balance reactivity with stability, opening up pathways that stretched well past routine synthetic work. Dozens of original patents and experimental articles mapped out the path for industrial production and intensive chemical research, which shaped how people think about this compound today in professional settings.
1-(2-Furoyl)Piperazine serves as more than just another entry in chemical catalogs—it participates directly in modern drug discovery and advanced chemical synthesis. Its structure, formed by linking a 2-furoyl group with a piperazine ring, brings a combination of aromaticity and basicity rarely matched in other intermediates. In my own work with medicinal chemistry projects, its appeal comes from a mix of stability, reactivity, and the doors it opens for further derivatization. Researchers and commercial buyers look at this compound not only for what it is, but for what it helps create down the line: heterocycle-rich candidates for pharmaceuticals, agricultural protectants, and specialty chemicals tailored for precise industrial targets.
1-(2-Furoyl)Piperazine typically comes as a solid, either white or near-off white. The sight of these fine powders in the lab always signaled a stable, manageable intermediate, nothing like the sticky gums or volatile liquids that cause headaches in small-scale synthesis. With a molecular weight hovering around 178 grams per mole and a boiling point that puts it well past room temperature, stability during handling remains solid. It dissolves in most polar organic solvents like ethanol, methanol, or dimethyl sulfoxide, but never really enjoys the company of water. The piperazine ring lends a basic character to the molecule, but the presence of the furan group dials down the alkalinity a notch, creating a reliable balance that avoids the worst of instability or over-reactivity during handling.
Labels and documentation always push for clarity about purity, moisture level, and storage guidelines—no sharpshooting required. Most commercial offerings provide purity above 98%, as even a slight drop can mess with reaction outcomes in pharmaceutical settings. Analysts rely on HPLC, NMR, and sometimes GC-MS to confirm identity and nail down impurity profiles, not because the compound likes to hide its flaws, but because even the tiniest contaminant might change a drug candidate’s fate. Storage tends to focus on dry, cool environments, avoiding the kind of prolonged light or air exposure that can erode chemical integrity. Labels shout out lot numbers, synthesis dates, and safety notifications, just to keep everyone honest during regulatory audits or quality reviews.
Synthetic chemists build 1-(2-Furoyl)Piperazine through a targeted approach. Starting with 2-furoyl chloride and piperazine, the preparation follows a classic acylation reaction, where the furoyl moiety latches onto one of the piperazine nitrogens. I’ve run a similar acylation on the benchtop, using mild base in anhydrous conditions with careful control over temperatures and dropwise addition to avoid runaway reactions. After that, the mixture moves through filtration and crystallization to yank out pure 1-(2-Furoyl)Piperazine, sometimes followed by washes and drying under vacuum to banish unwanted byproducts or moisture. The process captures the pragmatic spirit of chemical synthesis, stripping away excess fuss and focusing on reproducibility and yield.
Having such a reactive nitrogen on the piperazine ring, along with the electron-rich furan, gives this compound both flexibility and a readiness for modification. I’ve seen it used as a scaffold in nitration, halogenation, and even metal-catalyzed coupling reactions—reactions that chase new molecular architectures while preserving core stability. Chemists often “decorate” the piperazine or the furan ring to dial in bioactivity or solubility, and sometimes they build out more complex heterocycles by further cyclization. Its predictable reactivity under various conditions makes it a ready choice for innovating new analogs with subtle tweaks in function and performance.
People don’t always stick to just one name when referring to this compound, leading to a string of synonyms in literature and catalogs. It crops up as 1-(Furan-2-carbonyl)piperazine, 2-Furoylpiperazine, and sometimes even by internally assigned numbers in pharma research. In my experience, catalog numbers and CAS registry identifiers matter a lot more than legacy names once you get down to ordering, as they flatten out confusion and guarantee you’re getting what the synthetic scheme calls for. Some chemical houses even assign their own labels or abbreviations, which drift in and out of favor depending on the project or country.
Every time I’ve opened a bottle of 1-(2-Furoyl)Piperazine, the safety protocol lines up with best practices from occupational health and chemical hygiene plans. Eye protection, gloves, sometimes even working behind a fume hood—these steps feel routine but necessary, given unknowns about sensitization or irritation. Safety data sheets outline risk phrases and first-aid measures focusing on respiratory tract and skin contact. Disposal goes into designated chemical waste, never down the drain or in regular trash, since breakdown products and run-off to the environment raise red flags. Everyone in the lab, from students to senior staff, learns quickly that clear labeling and physical barriers keep incidents rare and manageable, a lesson hammered in every time someone cuts a corner and ends up with an irritant splash or inhalation scare.
People use 1-(2-Furoyl)Piperazine in medicinal chemistry as a key intermediate in the search for new therapies. Scientists graft it onto candidate molecules chasing everything from antifungal to anticancer outcomes, making it a reliable molecular pivot point in drug development. Agrochemical researchers dig into it for crop protection agents, formulating new active compounds to manage pests while sidestepping regulatory hurdles around old, overused modes of action. The versatility doesn’t end with drugs—people working on specialty coatings or niche materials sometimes insert this compound into their workflows to check for property enhancements. Those in analytical chemistry also chase after it as a reference standard or a tool for fine-tuning detection methods in chromatographic analysis.
Most of what makes 1-(2-Furoyl)Piperazine exciting on the research front comes from its molecular adaptability. Drug discovery teams lean on it as a launching pad for new pharmacophores, often participating in combinatorial libraries that test many variants at once. My colleagues in academic labs scan the literature for modifications off this core, looking for ways to swap in electron-donating or withdrawing groups on the furan ring and test SAR impacts. The pace of publication and patenting in this area confirms a robust appetite for incremental progress, even in the face of tough competition from other heterocycles. The need for new antiparasitic, antipsychotic, or neuroprotective compounds helps keep interest high, and the mix of industrial and basic science projects pulls in multidisciplinary teams.
No one likes to work in the dark with unknown toxicities, especially when the stakes include occupational health. Most screening runs for 1-(2-Furoyl)Piperazine point toward low to moderate acute toxicity, but there’s still uneven reporting in long-term effects or environmental impact. In vitro studies suggest that the piperazine backbone, unless heavily modified, avoids severe cytotoxicity at relevant concentrations. But animal tests remind us always to move carefully—just because acute exposure looks mild doesn’t guarantee the same for repeated or chronic exposure. My own risk assessments lean heavily on safety at the bench but keep a wary eye on what gets released downstream or lingers on surfaces. Ongoing efforts in toxicity profiling keep regulators, researchers, and production teams in the loop, feeding back new data to ensure regulations and best practices don’t lag behind emerging science.
If my experience tracking chemical trends holds, the horizon for 1-(2-Furoyl)Piperazine won’t shrink any time soon. Breakthroughs in computer-aided drug design keep highlighting the potential of flexible heterocyclic scaffolds—especially those already vetted through safety and processability screens. Environmental safety and sustainable chemistry will push for greener synthesis and smarter waste management, nudging manufacturers toward process improvements and alternative reagents. Applications in next-generation materials science, targeting everything from sensors to bioactive films, stand to expand as researchers unlock new properties of substituted furan-piperazine hybrids. Young chemists entering the field bring fresh ideas, mixing old methods with modern tools, and as they dive deep into the compound’s nuances, they uncover new connections shaping tomorrow’s chemical landscape.
Step into any research chemistry lab, and sooner or later, you notice bottles and vials marked with names like 1-(2-Furoyl)Piperazine. Not exactly a substance people talk about over breakfast, but for scientists working in drug discovery, that name means the door is open to new possibilities.
1-(2-Furoyl)Piperazine isn’t a consumer product. Instead, chemists use it as part of the search for new medications. The molecule combines two structures—furoyl and piperazine. Piperazine rings have cropped up in several drug classes, including antipsychotics and antiparasitic agents. The distinct furoyl group, which comes from furan (a ring containing oxygen), brings its own set of chemical behaviors. By joining these two, researchers create new starting points for building molecules with potential medicinal effects.
Many years of trying to turn basic chemistry into real-world treatments have shown that some groups of atoms change how a drug moves through the body or how strongly it attaches to targets in the body, like receptors or enzymes. Scientists keep testing new combinations, hoping to find one that makes a difference for people dealing with difficult diseases.
Most compounds like 1-(2-Furoyl)Piperazine will never reach a pharmacy shelf. A tiny fraction of molecules screened in labs each year survive the tough hurdles—activity, safety, and economics. Still, labs run thousands of experiments to figure out which molecular sketches deserve to go further in the journey.
For example, published research has shown scientists using this compound as a building block to explore anti-cancer and anti-inflammatory properties. Early studies sometimes note how the addition of a furoyl group affects the way the parent molecule interacts with disease-related proteins. In those scientific papers, researchers often describe a structure-activity relationship (SAR) approach. They tweak small parts of a molecule—like adding the furoyl group to piperazine—to see if the resulting change brings a stronger, safer, or more effective result.
Sometimes, chemical names turn up online in the context of new psychoactive substances or so-called “research chemicals.” It’s worth stating that 1-(2-Furoyl)Piperazine does not belong to commonly used recreational drug classes. Most evidence so far links this compound mainly to legitimate research uses. That said, regulating agencies keep a close watch on chemicals entering the market, checking for any sign of danger or uncontrolled use.
People sometimes worry about new chemicals for good reason. Data from the Centers for Disease Control and Prevention show that monitoring emerging chemicals helps protect public health, especially given the pace of synthetic drug development. Open communication between scientists, doctors, and the wider community keeps potentially risky substances from becoming a silent threat.
A strong collaboration between academic labs, pharmaceutical companies, and oversight agencies brings the best path forward. Sharing early information about compounds, such as safety profiles and biological effects, stops problems before they start. Proper labeling, handling protocols, and secure tracking of chemicals limit chances for misuse.
1-(2-Furoyl)Piperazine’s story really highlights the careful balance between innovation and responsibility. Careful research can shape better treatments. At the same time, strict monitoring keeps our communities safe as new molecules work their way from lab to life-changing medicine—or, as often happens, back to the drawing board.
People ask about the legality of chemicals like 1-(2-Furoyl)Piperazine for good reason. Over the past decade, research compounds have flooded websites all around the world. Sellers slap on the “not for human consumption” label and tuck their details away in privacy policies, but researchers, hobbyists, and even some people chasing a high have shown interest. Few ask what happens if the law changes while a compound sits in the postal system. I’ve seen the confusion first-hand, especially in online communities that orbit behind-the-scenes chemistry labs.
Ask a lawyer or a DEA agent about 1-(2-Furoyl)Piperazine and they’ll likely have to dig into their documents before answering. Not every state or country has a clearly written line on this compound. Right now, several nations treat every member of the piperazine family as fair game for regulation. For instance, BZP, which is a close cousin, ended up controlled in the U.S., New Zealand, and much of Europe. Laws targeting analogues often sweep up their chemical neighbors if they’re seen as “reasonably similar.” That means you can run a chemistry check, but the law uses broader strokes, catching substances that “look like” or “act like” banned drugs.
Just because a vendor offers 1-(2-Furoyl)Piperazine doesn’t mean cops or customs look the other way. Some countries use the Federal Analogue Act. In the United States, if a compound resembles a controlled substance and someone sells it for ingestion, legal troubles can start fast. For personal research in Europe or Asia, people find themselves thumbing through legalese and calling customs to double-check lists, which can change without warning. Even legal labs have watched customs seize their “research material” because it gave off the wrong impression.
I’ve lost count of reports from folks who took risks after reading on a forum that a compound wasn’t scheduled yet. Legal risks don’t only depend on a government database. Prosecutors sometimes decide intent based on text messages, web searches, or even poorly worded emails to suppliers. Vendors selling 1-(2-Furoyl)Piperazine sometimes operate offshore. They hide behind cryptic refund policies and mix legal advice with advertising—hardly the ingredients for peace of mind. Customs officers don’t accept the fine print from these shops.
People who think about buying chemicals like these have to consider more than just what’s written on a label or a website FAQ. Legal status doesn’t freeze in place. Lawmakers and agencies respond quickly when reports of harm surface. A compound seen as “gray area” today could end up scheduled tomorrow, leaving buyers on the wrong side of law changes. Forums and chemistry groups echo with stories of unexpected legal bills and lost shipments.
Before buying, people should check both federal and local laws, search for any recent law enforcement actions, and consult legal professionals who follow these issues closely. I recommend reading news from reputable public health and regulatory agencies, not just user groups or stores with a stake in selling. If a substance’s legal status seems murky, walking away often costs less than risking fines, lost shipments, or worse.
No one wants to guess with their health, yet it’s hard to find reliable information on the dosage of something as specific as 1-(2-Furoyl)Piperazine (1-FPP). Not every compound sitting on a chemical catalog is meant for people, and 1-FPP stands firmly in that gray area. Its primary use has been for research, not for treating health conditions or boosting performance. So when someone searches for the right dose, there’s a huge gap in trustworthy, evidence-based guidance.
Looking at official sources, 1-(2-Furoyl)Piperazine hasn’t gone through the sort of clinical testing that medicines or vitamins do. No dosing recommendations show up in published FDA or EMA documents. No standard values appear in reputable medical databases or handbooks that guide health professionals. Even academic papers keep their distance.
This tells us something important. If scientists working under strict guidelines haven’t figured out what counts as safe or useful, it’s risky for anyone to try to self-experiment with a compound still sitting in the research stage. The absence of clinical trials means there’s no roadmap for effects, side effects, nor potential toxicity. Publishing dosage online without this research turns guesswork into danger.
The chemistry lab and the human body follow different sets of rules. Labs control variables like purity, storage, and reactivity. Human bodies don’t—each person’s genetics, health, medications, and even diet can alter how a substance behaves after swallowing it or absorbing it through the skin. With 1-FPP, purity often varies between suppliers. Even with standards on paper, you can't guarantee each batch matches the label. Sometimes products meant “for research only” end up used by people without any oversight. Cutting corners or skipping checks can lead to injury or worse.
Legally, 1-(2-Furoyl)Piperazine often sits in a tricky place. Many governments treat it as a “novel psychoactive substance” or designer chemical. This means it can be snapped up online until regulators notice. Eventually, a pattern shows up: hospitalizations, mysterious side effects, sometimes deaths attributed to new lab-made chemicals. Then the bans hit. At this point, the risks have already crossed into real people’s lives.
Instead of reaching for new substances, people looking for therapeutic effects can talk to healthcare professionals about approved treatments. Doctors and pharmacists have access to up-to-date, peer-reviewed research. For anyone in a tough spot—stuck waiting on answers or tempted by shortcuts—there’s strength in honest questions. Is more energy, anxiety relief, or focus truly worth a roll of the dice on something not meant for human consumption?
It’s much safer to avoid unregulated compounds altogether. If concerns won’t go away, proper channels exist: clinical trials, poison control centers, and licensed healthcare providers. Medical research keeps looking for better answers but leans on ethics and transparency for a reason. Trust in those rules has helped folks outlive past health fads and quack remedies.
Every year, unknown substances enter the market with big promises but little proof. Wise choices come from weighing risks, understanding the facts, and listening to experts who stick to evidence. No shortcut or new formula is worth putting hard-earned health at stake.
1-(2-Furoyl)Piperazine isn’t a household name. In labs or certain online circles, it draws attention because it’s part of a broader group — piperazine derivatives. Some are used in legitimate medical research. Others show up in party pills and experimental designer drugs. It sounds like a mouthful, but the story often starts with someone curious about new experiences or researchers hoping for the next big breakthrough.
Most people eyeing substances like this want to know: is it safe? You see scattered mentions of it in online forums, a few animal studies, and bits of unpublished work. Straight talk: no one has pulled together a big human safety profile for 1-(2-Furoyl)Piperazine. With so little actual data, guesses and comparisons creep in.
People have described mild stimulation, changes in mood, or even just headaches and queasy feelings after use. Quite a few mention next-day tiredness, trouble sleeping, and a cloudy feeling in the head. Jitters, stomach pain, and pounding heartbeat also come up. These match the rollercoaster of effects from other piperazine compounds like BZP and TFMPP. Both have drawn enough problems — nausea, dehydration, shakes, and sometimes more serious trouble — that many countries now restrict or ban their use.
Without years of formal studies or real-world tracking of users, nobody can guarantee safety. Unlike meds that clear a long path of dose-finding, safety checks, and clinical trials, compounds like this lean on rumor and risk. That’s not nitpicking. Piperazine derivatives sometimes push heart rate, raise blood pressure, and cause lasting anxiety or panic attacks. Liver and kidney strain might not show up right away.
The brain runs on a tight balance. Messing with serotonin, dopamine, or other neurotransmitters can throw off mood, sleep, or even basic decision-making. You probably won’t see a label or a warning from a trusted health authority. Junk from the underground market sometimes contains leftover solvents or other impurities, growing the risks even more. No two batches behave the same way.
There’s a way forward, but it’s not through shortcuts or guesswork. If somebody ever wants to see legitimate use or even clear data on risks, only time, research funding, and transparent reporting can help. On the user side, choosing to walk away from the unknowns is the only smart move until real proof rolls in. If curiosity ever turns into trouble — heart racing, stomach churning, or worse — don’t wait it out alone. Hit up a healthcare provider, even if embarrassment kicks in.
Crowdsourcing safety information helps a little, but nothing beats real science. Governments and labs need to stay sharp, monitor new substances, and push for open reporting of harms. Skirting the edges with flashy new compounds looks tempting sometimes, but personal health and trust in medicine build on more than rumors.
Long hours spent in the lab have taught me that chemical storage decisions carry real consequences. One little slip—an unlabeled bottle, a forgotten cap—and the risks pile up fast. 1-(2-Furoyl)Piperazine falls into that group where a bit of carelessness breeds trouble: decomposition, cross-contamination, lost material, or worse.
The furan ring in this molecule looks innocent, but exposure to moist air or oxygen can prompt gradual breakdown. Humidity walks right past basic plastic covers, so I always stash this compound in tightly sealed glassware. Desiccators filled with silica gel keep stray moisture from creeping in. If stored at room conditions, even brief air exposure during handling can trim shelf life, so I lean toward using nitrogen or argon to blanket the container during storage.
Fluctuating temps give organic molecules a tough time. Cooling the storage space—think 2–8°C for short periods—helps slow reactions that steal purity and yield. I avoid deep freezing unless long-term preservation is the main goal, since rapid cycling between cold and ambient creates condensation inside bottles. Once water droplets sneak in, it’s game over for keeping quality consistent. Consistent, moderate cooling in a dedicated refrigerator usually does the trick.
Anyone with a sun-bleached textbook knows how fast light can damage things. Furan-based compounds react to visible and UV rays—chemical structures rearrange or degrade over time. Amber glass containers work best, and shelves far from direct sunlight or even intense overhead lights help prevent slow, invisible damage. It seems simple, but I’ve pulled more than one yellowed vial from a forgotten bench corner and wished I’d taken this advice to heart.
Clear, honest labeling remains the unsung hero of any lab. Writing batch number, date of receipt, and expiration goes a long way. With 1-(2-Furoyl)Piperazine, I treat every bottle as a possible hazard and keep storage spaces locked away from casual access. Even among colleagues, access protocols and inventory logs help spot problems before they cause bigger headaches.
Sometimes, compounds reach their end of life or degrade despite best efforts. Planning for disposal from day one saves grief. I collect expired or discolored material in clearly designated containers and flag them for hazardous waste pickup according to local regulation. The days of pouring leftovers down the drain are long gone. Environmental stewardship and personal safety connect through every step of chemical management.
No matter how many years pass, safe storage remains a moving target. Regulations change, new containers come along, and older habits give way to better ones. I keep one eye on reputable sources—SDS documents, peer-reviewed articles, manufacturer recommendations—and consult colleagues to cross-check my own practices. Real-world experience, plus up-to-date research, closes the gap on chemical safety. It’s an old lesson, but one worth repeating for 1-(2-Furoyl)Piperazine and every other specialty reagent on the shelf.
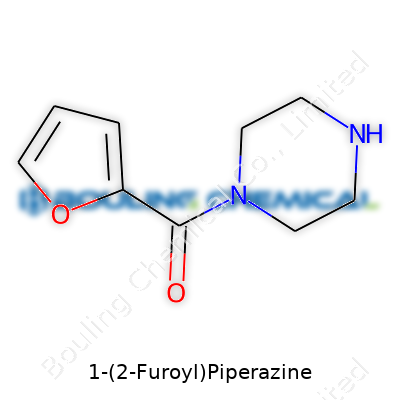

| Names | |
| Preferred IUPAC name | 1-(furan-2-carbonyl)piperazine |
| Other names |
1-Furancarbonylpiperazine 1-(Furan-2-carbonyl)piperazine |
| Pronunciation | /waɪn-tuː ˈfjʊə.rɔɪl paɪˈpɛr.əˌziːn/ |
| Identifiers | |
| CAS Number | [34727-10-1] |
| Beilstein Reference | 143550 |
| ChEBI | CHEBI:77983 |
| ChEMBL | CHEMBL38133 |
| ChemSpider | 22200280 |
| DrugBank | DB08226 |
| ECHA InfoCard | 03c6b3a8-5019-4fd1-a7f0-6d8a0de4fd7b |
| EC Number | 620-156-9 |
| Gmelin Reference | 104927 |
| KEGG | C14768 |
| MeSH | D059220 |
| PubChem CID | 125781 |
| RTECS number | GR9800000 |
| UNII | E38B75C92J |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DTXSID8022340 |
| Properties | |
| Chemical formula | C9H12N2O2 |
| Molar mass | 210.24 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.18 g/cm3 |
| Solubility in water | sparingly soluble |
| log P | 0.02 |
| Vapor pressure | 5.04E-3 mmHg at 25°C |
| Acidity (pKa) | 8.90 |
| Basicity (pKb) | 2.81 |
| Magnetic susceptibility (χ) | -63.73×10^-6 cm³/mol |
| Refractive index (nD) | 1.584 |
| Dipole moment | 2.78 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 437.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2026 kJ/mol |
| Pharmacology | |
| ATC code | N05CM21 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Harmful if swallowed. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| Flash point | 61.2°C |
| Lethal dose or concentration | LD50 (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50: 1600 mg/kg (rat, oral) |
| NIOSH | SN8800000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
1-(2-Furoyl)-4-methylpiperazine 1-(2-Furoyl)-4-phenylpiperazine 1-(2-Furoyl)piperidine 1-(2-Furoyl)morpholine 1-(2-Furoyl)pyrrolidine |