Back in the early twentieth century, folks working in both the dye and pharmaceutical fields noticed that imidazoles had a knack for popping up just about anywhere. Chemists started tinkering with methyl groups on the imidazole ring to see what new properties they could dig up. Sometime around the mid-1900s, 1,2-dimethylimidazole entered the picture. Its double methyl arrangement brought both researchers and manufacturers a fresh set of tools for tweaking reactivity in a range of complicated syntheses. Over decades, labs referenced it for making both simple intermediates and more elaborate functional materials, drawing on lessons from other alkylated imidazoles. The story of this chemical tracks closely with the overall growth in precision organic synthesis—one where small changes in structure can completely change the game.
1,2-Dimethylimidazole sits on many chemical stockroom shelves today for good reason. It serves not just as a reagent, but also as a building block for things as diverse as catalysts, antioxidant agents, and corrosion inhibitors. Both research outfits and big-name chemical suppliers offer it in various grades targeting pharmaceutical, materials, and advanced electronics sectors. In real-world practice—from my own hands-on experience with research on heterocycles—reliability and availability stack up as critical. Any unexpected interruption in supply will delay scale-up efforts, slow down catalyst development, and get in the way of new polymer research.
Looking at 1,2-dimethylimidazole as a physical specimen, most people find an oily, colorless-to-pale yellow liquid at room temperature. Its melting point hangs a bit below 0°C, with boiling coming in just under 200°C. Those methyl groups lower water solubility compared with plain imidazole but let it dissolve well in many routine organic solvents. The material always carries an unmistakable, pungent amine-like odor you won’t forget once you’ve worked with it in the lab. Its density and refractive index line up with similar small heterocyclic compounds. Chemically, that double methylation ramps up hydrophobicity while keeping the N-H group reactivity at bay—offering synthetic chemists more flexibility in reaction design. With a low vapor pressure and stable shelf life, it can be stored without much drama, though it will pick up water and dust if left open on the bench.
Manufacturers like to supply it at a purity of 98% or greater, with precise HPLC or GC analysis listed right on the spec sheet. SDS sheets flag it as a flammable, slight skin irritant, and not a substance you want to handle without gloves or eye protection. Individual lots get labeled with exact batch numbers and chemical identification codes, a big plus for documenting research and ensuring traceability. The CAS number—1739-84-0—serves as the ultimate short-hand identifier in any database or ordering system. Labels show hazard pictograms and storage advice: keep in a cool, dry place, away from strong oxidizers, and well away from anything that could ignite a flammable vapor.
The classic way to make 1,2-dimethylimidazole relies on methylation of imidazole, but routes differ depending on what’s in the starting line-up. A common approach uses methyl iodide with imidazole and a strong base to direct alkylation at both nitrogen atoms, but yields and selectivity can turn into an art form in practice. Another route, more attractive at large scale, employs formaldehyde and a methyl amine source under acidic conditions, making it easier to process without fiddly and expensive methyl iodide. From first-hand trial and error in organic labs, reaction order, temperature control, and choice of solvent matter a great deal. Poor planning will clog up columns with by-products or give frustrating mixtures. Purification usually turns to distillation or column chromatography, guided by TLC, and plenty of patience.
Chemists value 1,2-dimethylimidazole because those methyl groups change how it reacts without killing its ability to coordinate metals or act as a nucleophile. For example, in catalysis, its bulk and electron donating power boost efficiency in copper-catalyzed reactions—think of techniques like “click chemistry.” Modifications range from halogenation at the aromatic ring, oxidation to N-oxides, or even quaternization if further methylation is needed. I’ve worked with it as a ligand to tweak transition metal reactivity, and it’s surprising how deeply a methyl group on the right spot tweaks outcomes. It holds up well during processes involving mild acids or bases, but strong oxidizers or reducing agents shred its delicate ring.
Walk into any well-stocked chemical supply room or comb through research literature, and you’ll see this chemical pop up under different names. Some of the most common are 1,2-dimethylimidazole, N1,N2-dimethylimidazole, and 1,2-dimethyl-1H-imidazole. Other catalogs shorten the label to DMI or DMIM. Sometimes manufacturers tack on identifiers for analytical or purity grade, but the core chemical stays the same. The CAS registry number keeps everyone speaking the same language even if product names wander.
Lab safety training always stresses watching out for skin and eye contact, good ventilation, and keeping open flames far away. 1,2-Dimethylimidazole vapor can irritate mucous membranes, so fume hoods and gloves aren’t optional. Stock bottles usually come in amber glass, as the material is sensitive to light over time. Disposal routes follow local hazardous chemical rules, mixing with non-halogenated solvent waste. Professional work environments require GHS-compliant safety labeling, spill management kits, and full incident reporting. My time in both academic and industrial labs made clear that shortcuts—no matter how tempting—bring risk that isn’t worth it, especially with solvents or alkylating reagents on the same bench.
The reach of 1,2-dimethylimidazole cuts across chemistry, materials science, and even high-end electronics. In catalysis, it acts as a ligand in transition metal complexes, often improving yields in synthetic reactions for pharmaceuticals, fine chemicals, or specialty polymers. Electronics manufacturers use derivatives of this imidazole in anti-corrosion coatings for circuit boards, tapping into its chemical stability and hydrophobicity. More recently, the compound found a spot as a building block in some ionic liquids, supporting greener solvents and next-generation battery research. In the petroleum sector, it shows up as an additive to fight corrosion in complex pipe systems.
Active research projects are pushing the boundaries of how such simple methylated imidazoles can improve modern technology, especially in catalysis, electrochemistry, and polymer science. Surface chemists test 1,2-dimethylimidazole-based compounds to find better mediators and stabilizers for nanoparticles. Pharmaceutical teams look at its performance in drug discovery, where metabolic stability and bioavailability data steer tweaking of promising molecules. In green chemistry, this compound gets a fresh look for ionic liquids and deep eutectic solvents used in environmentally friendly synthetic techniques. My own experience suggests that access to well-characterized, high-purity samples lays the groundwork for meaningful, reproducible results—cutting down on wild goose chases caused by sneaky impurities.
Toxicology data on 1,2-dimethylimidazole reveal that it carries moderate risk if handled improperly, with irritation to skin, eyes, and respiratory tract sitting at the top of the list. Animal studies show acute toxicity only at doses far beyond what a worker or lab user would usually encounter. Chronic exposure data remain limited, putting more importance on strong workplace protections and responsible reporting of incidents. Regulatory agencies flag it as a material needing proper risk assessment, especially in places like drug production or electronics manufacturing, where operators use larger quantities. Thorough risk assessments, reference doses, and existing occupational safety data ensure transparent handling—lessons worth remembering as new, related chemicals enter the market.
With interest growing in both tailored organic synthesis and sustainable technologies, 1,2-dimethylimidazole gains attention from folks working in everything from battery research to advanced polymer design. As processes get greener and call for new solvents and catalysts, demand rises for molecules that deliver performance with fewer environmental headaches. Better pathways for its synthesis, improvements in waste reduction, and more robust toxicology studies stand to benefit both industry and academia. Given its proven role in lab and factory, any advances in cost, safety, or performance will echo through fields as different as energy storage, medicine, and advanced electronics. Researchers, engineers, and safety officers have their work cut out navigating both legacy uses and novel frontiers for this small but mighty molecule.
1.2-Dimethylimidazole might sound like a mouthful, but its uses stretch far beyond a laboratory shelf. Chemists and manufacturers don’t just treat this compound as a background player — it helps unlock better materials, smarter processes, and even more sustainable products. In my own lab days, I'd sometimes spot a bottle of 1.2-Dimethylimidazole stashed away on the shelf, a silent helper in all sorts of hidden reactions.
Plastics and resins probably wouldn’t be as tough or durable without compounds like 1.2-Dimethylimidazole in the mix. It steps into the scene as a catalyst, speeding up the process that transforms small building blocks into robust polymers. In epoxy resins, for example, it takes on the role of a curing agent. This means faster setting times and bonds that actually last. Electronics fabricators lean on it to produce circuit boards that resist both heat and stress. My friends working in coatings and adhesives often mention how one tweak in the catalyst selection — swapping in 1.2-Dimethylimidazole — can mean the difference between a cracked finish and one that holds up under years of sunlight and rain.
Chemistry often comes down to getting the right reaction in the right conditions, and this is especially true in drug manufacturing. 1.2-Dimethylimidazole helps pharmaceutical chemists drive reactions toward cleaner results. Its role as a base or a nucleophile can simplify purification, which keeps costly waste down. In an industry where every percentage point in yield counts, choosing a helping hand like this compound can keep manufacturing both lean and green. The search for efficiency in drug synthesis never stops; seeing 1.2-Dimethylimidazole crop up in patent filings tells me how valued it is across the globe.
Safety can’t take a back seat, especially in buildings packed with electronics or synthetic textiles. 1.2-Dimethylimidazole plays its part by combining with other chemicals to boost thermal resistance in foams and coatings. Many fire-safe building materials rely on exactly this sort of chemistry, where a single additive makes the difference during those precious minutes of a fire. Having worked with fire safety standards before, I know how regulatory pressures push innovation — and this molecule shows up time and again in those new recipes for safer homes and workplaces.
Manufacturers need to rethink their formulas as environmental concerns mount. Instead of sticking to older, harsher chemicals, many have adopted 1.2-Dimethylimidazole because it improves reaction efficiency, which can mean lower energy use. Companies wanting to cut both costs and their environmental footprint have found this compound useful in greener coatings and advanced materials. I’ve watched small start-ups pitch new, eco-forward paints and plastics, and this ingredient features prominently as a way to balance innovation with responsibility.
The hidden chemistry behind our everyday items depends on small tweaks and discoveries. 1.2-Dimethylimidazole gets jobs done, but it’s not a cure-all. Supply chain hiccups and safety handling still pose challenges. Some researchers are now searching for ways to recover or reuse this and similar compounds during manufacturing. Incentives that encourage recycling chemical auxiliaries could keep future chemical plants both profitable and less polluting. If more labs collaborate, sharing methods that save energy or enable recycling, we could sharpen the green edge of the chemical industry. For now, though, 1.2-Dimethylimidazole keeps showing how one molecule can punch above its weight in making products safer, cleaner, and more reliable.
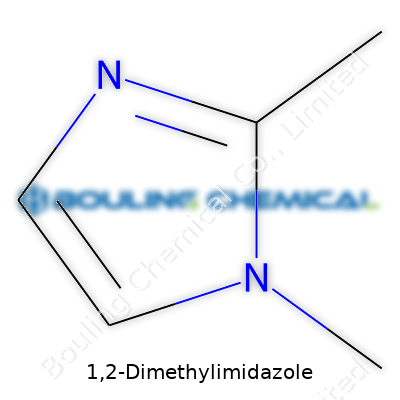
For anyone navigating through organic chemistry, recognizing the structure and properties of key molecules often opens doors. Take 1,2-Dimethylimidazole, for example. Its chemical formula stands as C5H8N2, representing five carbon atoms, eight hydrogens, and two nitrogens arranged around an imidazole ring. Weighing in, its molecular weight measures at roughly 96.13 g/mol. These numbers look small, yet the role this compound plays in research and industry punches above its weight class.
I've seen plenty of chemists get tripped up by minor changes in structure leading to huge differences in activity. Adding two methyl groups—here at the 1 and 2 positions on the imidazole ring—can flip an everyday molecule into something special. Those methyl groups seem trivial, but they shift the electronic environment, hue the molecule’s reactivity, and even change how it binds with other molecules. At the bench, this adjustment can spell the difference between a reaction working and stalling out.
Some might glance at a formula like C5H8N2 and wonder where it all fits in outside the lab. I’ve found that these tiny tweaks in molecular structure often lead to big real-world advances. For instance, imidazole derivatives show up all over drug development, acting as the backbone for antifungal meds and enzyme inhibitors. The pharmaceutical world relies on subtlety: slightly shifting a functional group has resulted in whole patents. Given the explosion of interest in catalyst design, 1,2-dimethylimidazole makes the rounds as a ligand, changing the fate of catalytic cycles and boosting efficiency. These aren’t abstract wins; they trickle down to cheaper, better medicines and greener chemical processes.
Even outside medicine, the molecule catches the attention of materials scientists. Researchers tap imidazole rings to develop ionic liquids and next-generation electrolytes for batteries. This particular derivative, with its specific methylation, gives fine control over properties like melting point and solubility. For energy storage, those tweaks might stretch the life of a battery or push up its charge capacity. Every time a new electrolyte gets tested, small compounds like this one play their role.
Yet it isn’t all smooth sailing. Producing 1,2-Dimethylimidazole on scale heads straight into the usual headaches: cost, purity, and environmental footprint. Solvent use, waste disposal, and the hunt for green synthesis routes stay on everyone’s agenda. Some labs use catalytic methylation, others lean on older, less selective methods. If chemistry can produce this compound efficiently, the knock-on effects touch everything—better catalysts, less pollution, and lower production costs.
One way forward involves nailing down greener synthesis. I’ve worked with enzymatic systems that show real promise, attaching methyl groups with less waste and smaller energy bills. Other times, chemists have adopted new methods that shrink hazardous byproducts. More access to high-quality 1,2-dimethylimidazole opens up new research projects and helps everyone chasing better batteries, greener reactors, and smarter drugs. This single molecule doesn’t carry the weight of the world, but its chemical footprint finds its way into the hands of real people—sometimes without them noticing.
Chemistry classes make a big deal out of formulae and weights, but the real magic happens outside the chalkboard. 1,2-Dimethylimidazole—known by a formula as plain as C5H8N2, weighing 96.13 g/mol—steps into the story every time researchers bet on smarter, healthier, and more sustainable choices.
Open a drum of 1.2-Dimethylimidazole, and you catch a whiff that tells you this isn’t something for an open shelf next to the coffee machine. Over the years, I’ve watched folks get a little too comfortable around chemicals. Familiarity leads to taking shortcuts. It’s easy to forget how quickly a minor slip-up with certain compounds makes for a serious mess—or a trip to the emergency room.
Back in my early lab days, the old hands drilled storage rules into us, not because a poster told them to, but because no one wants to be the reason for a panic alarm or worse. The first rule with 1.2-Dimethylimidazole—don’t set it near the heat. This chemical’s flash point sits lower than a lot of folks think, and exposure to an ignition source raises the risk of fire. We always used cool, dry storage, and checked the label on the bottle at least twice.
Humidity creeps up in most rooms, especially during the hot months. A sealed, dedicated cabinet with desiccant packs keeps moisture out and keeps the chemical stable. Any container, if not closed up tight, lets the contents pull water from the air, which only leads to clumping or unwanted reaction. Never once did I see a professional store this chemical in glass containers with questionable lids. Polyethylene bottles with screw caps always did the job better.
We all know someone who shrugs off gloves with the excuse that they’re in a rush, or goggles because “it’s just a quick pour.” That’s how accidents start. I still remember a coworker getting a tiny splash on the skin—redness turned to burning in less than a minute. Nitrile gloves, safety goggles, and a lab coat became my second skin every time I went near materials like this. My lungs are grateful we used the fume hood for weighing and transferring. Even a brief breath of the vapor feels rough.
Spills always got strict treatment: clean up right away, ventilate, and double-bag the waste. We never swept things under the rug. Every cleanup called for an absorbent material, and once, a spill wiped up hurriedly led to nasty stains that tormented the next person to use the bench.
Training remains the backbone of good practice. Regular drills on storage and handling reduce mistakes, even for veterans. Supervisors and managers, in my own experience, get better results by leading by example. No lectures—just putting on the right PPE first and showing new techs how to double-check containers.
Good signage goes a long way, too. A clear “flammable, keep cool, keep dry” sign, written in large letters and posted at eye level, beats hidden safety data sheets by a mile. Suppliers have gotten better about printing pictograms that take away the language barrier, but keeping the workplace culture focused on safety matters more than any printout.
Most problems come from hurrying or from ignoring warning signs. Labeling containers clearly, keeping incompatible chemicals apart, and keeping a spill kit within arm’s reach all keep bad days at bay. There’s no shame in rereading the safety data sheets or even double-checking the cupboard door before leaving the room. In the end, a little effort keeps everyone heading home in the same shape they arrived.
Spending time in a lab means you start to notice which chemicals draw folks together for a safety huddle. 1.2-Dimethylimidazole deserves a spot on that list. This compound doesn’t glow orange or send up warning fumes, but its hazards command respect. It smells fishy—literally—and a sharp nose notices its vapor way before seeing the clear liquid spilling over. Anyone who’s cracked open a container knows quickly: ignoring basic safety leads to immediate regret.
Plenty of people slip gloves on and barely think about why. I learned quick that running bare-handed into 1.2-Dimethylimidazole means splitting time between the rinse station and a health check. It soaks through the skin, and irritation hits faster than you expect, causing redness and sometimes more. Eyes turn into the real emergency. Splash even a little, and you spend the afternoon flushing out pain, not your science experiment.
A lab gets stuffy sometimes. Bottles like this make those moments risky. Breathing in vapors doesn’t just burn the nose or throat; with enough exposure, headaches roll in, and you start getting dizzy. Nobody loves laboring under a fume hood, but with chemicals like this one, that big fan overhead saves jobs and sometimes much more.
It’s one thing to keep flammable items away from candles, another thing to realize some aren’t so obvious. 1.2-Dimethylimidazole catches fire at a relatively low temperature. The liquid ignites with surprising speed—static sparks, open flames, or even a hot plate left unattended can turn a routine procedure into a panic. Real stories fly around about bottles of the stuff flashing up without warning. It pays to double-check containers, clean up drips, and keep ignition sources far away.
One time, a friend left a bottle of 1.2-Dimethylimidazole outside the flammables cabinet, just overnight. The room reeked the next morning, and the supervisor nearly lost it. This kind of mistake isn’t harmless. Humid air, wild temperature swings, and contact with the wrong kind of plastic can eat away at containers and create spills. Securing it tightly, locking it out of reach, and logging each use keeps not only your own skin safe but everyone else’s too.
Where you throw away leftovers matters. Dumping 1.2-Dimethylimidazole down the sink doesn’t just break the rules. It poisons water and hurts the folks managing the waste. The right move is taking the time to send it off with other hazardous waste, clearly marked and ready for treatment. It takes coordination with environmental safety staff and means reading up on current rules—not trusting luck or memory.
Folks using 1.2-Dimethylimidazole need straightforward habits: protective gear, diligent labeling, solid ventilation, and common sense with fire risks. A culture of checking procedures beats relying on luck every time. Regular retraining keeps everyone sharp, especially as people come and go. Chemical safety isn’t a one-person job; it’s a promise you make to the whole team.
People working in research labs or chemical manufacturing know 1,2-Dimethylimidazole for its practical uses—often as a catalyst or building block in specialty chemicals and polymers. Things can get complicated fast when a batch turns out less pure than expected. Most buyers seek out material over 98%, sometimes reaching up to 99%. Lesser purity not only throws off reactions, but can also introduce byproducts tough to filter out. That gets expensive, chews up valuable time, and draws safety headaches.
Lab staff usually double-check the certificate of analysis before ordering, especially for projects needing tight controls. The difference between 97% and 99% can mean the failure of an entire batch, wasted work hours, and lost money. For those who have tried cleaning up after “good enough” chemicals mess up a reaction, paying a little more for higher purity starts to look like a bargain: less waste, fewer headaches, and more reproducible results.
Big chemical suppliers know their customers never want to buy more than they need or risk contamination by decanting out of containers too large for their operation. Packaging matters. Most common sizes range from small glass bottles for the analytical world—think 25 grams or 100 grams—up through 250-gram, 500-gram, and full kilogram lots.
On the industrial side, companies ordering for scale-up often want drums or jugs. Five-kilogram and 25-kilogram high-density poly containers guard against leaks and withstand rough handling. Glass bottles remain popular for purity’s sake, but can be awkward at kilogram scale; plastic wins out as weight climbs both for safety and practicality.
Shelf life always comes up. People unfamiliar with this chemical might be surprised to learn how reactive imidazoles can be to moisture and air. Packaging with tightly sealed lids, or even nitrogen-purged containers, limits degradation and keeps the product as close to its original specs as possible. Staff working in humid or coastal climates often ask for moisture-resistant sealing as an add-on.
From my own experience, opening a poorly sealed bulk container brings instant regret—clumpy, yellowed powder, wasted money, maybe a ruined project. Yanking a glass bottle from an air-conditioned storeroom only to find it sweating the moment it hits the bench shows why proper packaging isn’t an afterthought. Repackaging at the point of use may sound smart, but it introduces risk, and before you know it, quality slips.
Price and supplier reliability shape real-world decisions, far more than many like to admit. If a supplier returns consistent, high-purity product in well-sealed containers, most chemists and purchasers stick with them. Small mistakes in packaging during transit lead to losses that bottom-line-focused managers don’t forget. For those in smaller operations without warehouse facilities, suppliers offering multiple pack sizes and good shipping practices win business.
Some in the industry talk about eco-friendly, returnable containers or smart packaging with moisture sensors. These sound great in theory, but the old standbys—tight seals, carefully labeled bottles, and honest paperwork—still pay off day to day. For improvements, more education from suppliers about best storage practices and increased batch transparency build confidence and save resources in the long run.
In this market, purity and packaging shape the difference between smooth work and costly surprises. Buyers who remember this often save themselves trouble, cost, and frustration down the line.
| Names | |
| Preferred IUPAC name | 1,2-Dimethyl-1H-imidazole |
| Other names |
1,2-Dimethylimidazole 1,2-Dimethyl-1H-imidazole |
| Pronunciation | /ˈwaɪ.pəˌdiː.mɛθ.ɪl.ɪˌmɪd.əˈzoʊl/ |
| Identifiers | |
| CAS Number | 1739-84-0 |
| Beilstein Reference | 120924 |
| ChEBI | CHEBI:140564 |
| ChEMBL | CHEMBL12793 |
| ChemSpider | 19369 |
| DrugBank | DB02137 |
| ECHA InfoCard | 100.022.291 |
| EC Number | 211-996-7 |
| Gmelin Reference | 68295 |
| KEGG | C02172 |
| MeSH | D003187 |
| PubChem CID | 69989 |
| RTECS number | MK5250000 |
| UNII | L6W5E6KH9Y |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | DTXSID5036725 |
| Properties | |
| Chemical formula | C5H8N2 |
| Molar mass | 96.13 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | amine-like |
| Density | 0.94 g/mL |
| Solubility in water | miscible |
| log P | 0.26 |
| Vapor pressure | 0.34 mmHg (25°C) |
| Acidity (pKa) | pKa = 8.1 |
| Basicity (pKb) | 8.22 |
| Magnetic susceptibility (χ) | -35.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 1.05 mPa·s (25 °C) |
| Dipole moment | 1.83 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 159.0 J mol⁻¹ K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -20.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3763.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Harmful if inhaled. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P362+P364, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | > 74°C |
| Autoignition temperature | 355°C |
| Explosive limits | 4 - 32% |
| Lethal dose or concentration | LD50 oral rat 540 mg/kg |
| LD50 (median dose) | LD50 (median dose): 977 mg/kg (oral, rat) |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 mg/L |
| IDLH (Immediate danger) | 50 ppm |
| Related compounds | |
| Related compounds |
Imidazole Methylimidazole 2-Methylimidazole 4-Methylimidazole Ethylimidazole |