Chemists first looked at substituted piperidines over a hundred years ago, hoping to find new building blocks for the growing discipline of medicinal chemistry. As researchers noticed the unique combination of reactivity and stability in chloroethyl groups, they turned their attention to chloroethylpiperidinium compounds. Early work detailed possible applications in alkylating agents, which found their way into pharmaceuticals, chemical research, and even agriculture. During the mid-20th century, the field of organochlorine chemistry boomed, and universities in Europe and North America published data about chloroalkylated piperidines. Lab notebooks from that time show just how keen chemists were to find compounds balancing activity and manageable toxicity. This history guides ongoing work with 1-(2-Chloroethyl)Piperidinium Chloride.
1-(2-Chloroethyl)Piperidinium Chloride belongs to the class of quaternary ammonium salts, pairing a piperidinium ring with a reactive chloroethyl side chain. Chemists and product developers recognize this compound for its easy solubility in water and good storage stability in sealed containers, which matters in lab and industrial environments where consistent reagent quality counts. Suppliers typically provide the chemical either as a crystalline solid or as a ready-to-dissolve powder, packaged in containers that reduce moisture uptake. Having worked with chemical inventory systems myself, I’ve seen requests for pure samples arise in contexts ranging from organic synthesis to performance testing of functional materials, all drawn by this compound’s blend of reactivity and structure.
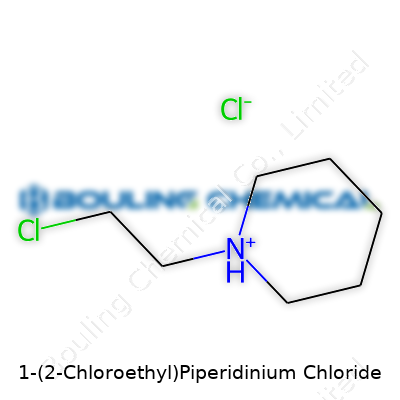
1-(2-Chloroethyl)Piperidinium Chloride generally appears as a pale white or off-white crystalline powder. It absorbs water from the air, so proper storage in tightly closed vessels becomes essential to prevent clumping or decomposition. Melting points usually fall between 180°C and 195°C. Solubility in cold and warm water is high, with even small concentrations dissolving quickly. The molecule combines an aliphatic six-membered ring with a hooked chloroethyl group. That reactive chlorine atom attracts attention from synthetic chemists seeking alkylation reactions, thanks to its ability to leave the molecule during reaction steps. In the environment or in the lab, this group’s relative lability means the compound only persists in conditions that don’t provoke nucleophilic substitution.
Manufacturers describe 1-(2-Chloroethyl)Piperidinium Chloride by purity, chloride content, and physical consistency. Labels include batch number, net mass, assay data, and the regulated hazard pictograms based on correct global standards such as GHS. Purity ranges usually hit 98% or higher, proven by titration, NMR, or HPLC, with moisture less than 1%. Every bottle’s label must carry the correct hazard statements, given the compound’s reactivity and irritant potential. Having managed chemical storerooms in academic labs, I know how crucial traceable labeling is for safety audits, incident reporting, and avoiding mix-up with nearly identical compounds.
Chemists typically synthesize 1-(2-Chloroethyl)Piperidinium Chloride by quaternizing piperidine with 2-chloroethyl chloride or a similar halide. The process runs under controlled temperatures, as both starting materials give off fumes and can form hazardous byproducts if heated too quickly or exposed to excess moisture. Simple glassware, such as round-bottom flasks and condensers, can do the job at scale for research, but controlled reactors help maintain consistent batch outcomes on an industrial scale. Any preparation of this compound means dealing with corrosive and hazardous chemicals. A fume hood and gloves come standard. In my own research experience, tracking batch yield and impurity content during this procedure makes the difference between a usable reagent and one that will complicate downstream experiments.
The active chloroethyl group in this molecule acts as an efficient alkylating site. Reactions with nucleophiles such as amines, thiols, or even some stabilized carbanions produce substituted derivatives or open the door to more complex structures. Advanced modifications harness the piperidinium core’s ability to stabilize positive charges while the leaving group departs. Chemists in medicinal research use this property to graft the unit onto candidate drugs, tuning molecular characteristics for absorption or cellular uptake. On the materials chemistry side, 1-(2-Chloroethyl)Piperidinium Chloride can link to polymers or crosslink resins. Each reaction must account for the reactive nature of the chloroethyl group, which can hydrolyze or react with plastic, metal, or glass under the wrong conditions. From personal experience working on cationic surfactant synthesis, even minor trace water in the mixture can disrupt the yield, so drying and purification require close attention.
Different suppliers list this compound under names such as 1-(2-Chloroethyl)piperidinium chloride, N-(2-Chloroethyl)piperidinium chloride, or by international registry numbers like CAS 38363-32-5. Some literature uses related names from earlier years, such as Chloroethylpiperidinium Quaternary Salt, or simply abbreviates it as CEP chloride in figures and methods sections. Staying alert to these synonyms aimed at tracking and ordering the right compound, especially given the potential for confusion with similar alkyl halide piperidines or even industrial cleaning agents that feature parallel nomenclature. Cross-referencing registry numbers has helped me resolve several supply chain mixups.
There’s no question that 1-(2-Chloroethyl)Piperidinium Chloride needs careful handling. Exposure can cause irritation of skin, eyes, and respiratory tract, and gloves plus goggles belong in the standard toolkit for anyone working with it. Larger quantities need sealed secondary containment and designated transport. The molecule’s reactivity commands proper segregation from strong bases, oxidizers, and reducing agents. Working with this compound reminds me of handling alkyl halides for cross-coupling reactions: one negligent move, and you’ve got a spill or a burned hand. Updated Safety Data Sheets (SDS) from reputable vendors keep lab group members aware of new handling recommendations, and any procedural change comes with retraining. In regulated settings, strict record-keeping and waste management tie together ethical stewardship of hazardous substances and safe working conditions.
Laboratories and pilot plants turn to 1-(2-Chloroethyl)Piperidinium Chloride for synthesis of active pharmaceutical intermediates, development of cationic surfactants, and research on advanced polymeric materials. Organic chemists value its alkylating abilities for building up designer molecules tailored for biological or materials testing. Drug discovery teams examine its effects on cellular models or use it as a starting point for molecules targeting ion channels or neurotransmitter systems. Material scientists sometimes incorporate this compound for tuning the behavior of ion-exchange membranes. I’ve seen it pop up in patents for antistatic agents and as one ingredient in proprietary adhesives and coatings. Its structure lets innovators test new chemical scaffolds without reinventing synthetic routes every time.
A fair bit of current R&D explores modifications of chloroethyl-piperidinium compounds to discover ones with better selectivity, lower toxicity, or unique bioactivity. Analysis happens through a mixture of in vitro assays, computational docking, and physicochemical testing. New synthesis pathways aim to minimize byproducts and enhance atom economy while reducing hazardous waste. As someone who’s worked in combinatorial synthesis labs, I see how minor tweaks to the piperidinium ring or the attached alkyl group can produce huge differences in downstream biological assays or chemical transformations. Collaborations between academic groups and industry partners spur the creation of libraries of derivatives, feeding new data into public databases and peer-reviewed journals. Interest grows in greener preparation methods and scalable purification strategies.
The presence of both the chloroethyl group and the piperidinium moiety means this molecule poses a range of health hazards. Lab animal studies point toward moderate acute toxicity, mainly from the alkylating action on proteins and DNA. Researchers check for cytotoxic effects before onboarding derivatives into drug screening programs. In occupational settings, chronic exposure can lead to respiratory irritation and possible neurological symptoms, so laboratory personnel rotate duties to limit time with this chemical. Environmental fate studies show decomposition products can disrupt aquatic ecosystems, so responsible waste disposal protocols follow strict regulation. Training lab members based on real-world incidents—accidental inhalation or skin contact—instills respect for the risks and appreciation for the stringent precautions the material demands.
With advances in synthetic chemistry, scientists continue modifying 1-(2-Chloroethyl)Piperidinium Chloride to solve current problems in pharmacology, material science, and manufacturing. Automation technologies promise better yield and fewer human errors, while ongoing green chemistry research offers the hope of fewer hazardous byproducts and safer working conditions. Regulatory shifts toward sustainability push chemical makers to re-engineer routes for both energy savings and waste minimization. There is strong academic interest in bioisosteric replacements for its chloroethyl group, aiming to reduce toxicity while preserving synthetic versatility. Based on the flood of patents and research papers, it’s clear the story of this compound pushes on, nudged forward by demands in medicines, smart materials, and fundamental chemistry.
I’ve come across many niche chemicals throughout my career, but 1-(2-chloroethyl)piperidinium chloride stands out. Folks in chemistry circles know it’s not something you’d use in a college lab, or let sit near curious hands. It’s a specialty compound—created and handled for only a few very strict applications. Unlike bulk chemicals used in manufacturing or cleaning, this one sticks to a narrow role: as a research tool, mostly in highly regulated environments.
Dig into the literature, and you find that 1-(2-chloroethyl)piperidinium chloride pops up in drug development work, especially for cancer therapeutics. It’s a derivative of piperidine, which has served as a backbone for hundreds of medicines. Chemists look at these compounds to see how they affect certain lines of cancer cells. Some researchers use it as a template to make even more selective agents that could target cancer while leaving healthy cells alone—a tough puzzle in oncology. A team in Germany published a paper last year, showing that tweaking this molecule changes how it interacts with DNA in a lab dish. They weren’t making pills; they were gathering clues.
This compound doesn’t end up in over-the-counter products. Regulators classify it as a controlled substance in several countries. They do this because it resembles chemical building blocks used in old chemotherapy drugs known for their extreme toxicity. If handled wrong, these compounds can damage living tissue quickly. That means anyone who works with them trains for months and follows layer upon layer of safety protocols—proper gloves, hoods, air filtration. It’s not like opening a jar of salt or even strong bleach.
Some folks ask why anyone bothers when the risks stack up so high. The answer lies in what scientists learn from studying potent compounds. By observing exactly how this molecule reacts with DNA, researchers can map out the pathways that cancer cells use to survive—or die. Every small discovery, even if it comes from a dangerous chemical, might one day lead to safer drugs. A breakthrough in the lab often starts with an unlikely substance that never leaves the test tube, but it pushes the field forward.
Institutions push for strict oversight. Labs dealing with 1-(2-Chloroethyl)piperidinium chloride need special permits. Transparent logs track its use and disposal, which protects communities from accidental leaks. Some universities have switched to simulator compounds—molecules that behave in a similar way with fewer risks—to cut down on accidental exposures. Funding agencies now demand risk assessments before research using these compounds moves forward.
It’s important to remember that the public judges chemical research not just by the discoveries, but by the honesty and safety behind them. Researchers who use compounds like 1-(2-chloroethyl)piperidinium chloride build trust by sharing safety data, updating disposal methods, and publishing all findings—good or bad. By supporting strict oversight and open communication, science keeps moving forward without putting people at unnecessary risk.
1-(2-Chloroethyl)Piperidinium chloride looks complicated on paper, but the core facts stay simple. The chemical formula stands as C7H16Cl2N, which tells us it has seven carbon atoms, sixteen hydrogen atoms, two chlorine atoms, and one nitrogen atom. You’ve probably seen molecular weights listed next to chemical names, and this one adds up to about 184.12 g/mol. Grabbing the right numbers matters for lab work, pharmacy compounding, and research, especially where even a small math error can cause confusion or flawed results.
Having those numbers—C7H16Cl2N and 184.12 g/mol—on your side helps a lot. Scientists and students use these values every day to weigh out doses, mix solutions, or figure out yields from reactions. I remember back in my first organic chemistry lab, just trying to work through stoichiometry without a reliable formula felt like walking blind. Any mistake could set back hours. Calculations start from straightforward data like formula and molecular weight, and skipping those details brings headaches, not breakthroughs.
In pharmaceutical labs, simple accuracy keeps things running safely and smoothly. In medicine, the right compound at the wrong dose risks patient safety. Lab techs and chemists trust in these fundamentals because they prevent errors at the bench and in production batches. During my work experience, tracking the tiniest decimal in a calculation protected everyone’s time and reputation—not just my own experiments. These numbers also steer automated equipment in quality control labs and factories, so accuracy ripples out way beyond textbooks and beakers.
Chlorine atoms in chemical structures often get a bad rap because they’re found in a lot of hazardous substances. But chlorine brings unique reactivity that leads to new treatment options and molecular research tools. The presence of a “2-chloroethyl” group, especially attached to piperidine structures, hints at biological activity—sometimes in cancer research, other times in neurological studies. At the same time, handling these molecules safely stays crucial, as they can be sensitive and require controlled environments.
Challenges pop up around chemical accessibility and safe handling. Smaller academic labs sometimes wrestle with outdated balances or poorly labeled containers, while larger facilities put their trust in automation and digital inventory systems. One solution I saw working well is keeping a regularly updated digital chemical inventory. Cross-referencing every entry with real-world labels seems simple, but it cuts down on mix-ups and keeps everyone safer.
For researchers without deep backgrounds in chemical nomenclature, access to clear databases like PubChem gives another layer of safety. They make it easier to cross-verify structures and weights before starting jobs in the lab. For the best lab management, having open conversations about chemical naming, weighing mistakes, and reliable double-check systems builds a culture that values accuracy and trust.
The foundation stones—chemical formula and molecular weight—set up every kind of chemistry work that follows. Precision, open access to reliable data, and safe handling protocols make sure research delivers safe, repeatable, and trustworthy results. Whether someone’s preparing a simple solution or developing the foundations for a new therapy, those numbers carry a practical weight. Getting them right means more than passing a quiz; it supports discovery, safety, and progress across the sciences.
Anyone with a chemistry background learns early that hazardous chemicals demand respect along with knowledge. I remember the first time I dealt with a volatile compound in my college lab—the reminder from my instructor couldn’t have been clearer: always check the storage details before even opening the bottle. The same goes for 1-(2-chloroethyl)piperidinium chloride. This chemical holds risks that matter, so treating storage as an afterthought could cause accidents or even ruin research.
Most chemicals with reactive or corrosive elements stay safer at lower room temperatures, and this one fits that pattern. Warm rooms speed up degradation and increase the risk of vapor or fume release. Cool, steady temperatures help maintain its stability. My training taught me to value the reliability of a climate-controlled cabinet—never leave such materials in a place where the air gets stuffy or the sun shines through a window.
Humidity isn’t a friend to sensitive compounds. Moisture can affect shelf life and sometimes trigger unwanted reactions. At the research institute where I spent a summer, plain silica gel packs lined every chemical cabinet. That simple measure kept humidity down, which is far more than a precaution; it actually stops chemical messes that would disrupt serious work.
At the heart of chemical safety sit two easy rules: keep incompatible substances far apart, and keep people protected. Never place this compound near strong oxidizers, acids, or flammable solvents. In one busy lab, I saw a disastrous mix-up with another alkylating agent because someone set it down next to a reactive shelf. Collection and labeling by hazard class isn’t bureaucracy—it’s the best insurance policy for your lab.
Personal safety gear deserves attention. Even in storage, leaks or spills happen. I keep chemical-resistant gloves and splash goggles at the ready. Anyone handling containers owes it to themselves to double-check seals before and after getting the chemical out. Good lids and clear codes on bottles make a world of difference for day-to-day safety.
Containers make or break safe storage practices. Glass works in most cases, especially borosilicate types that stand up well to many types of chemical stress. Polyethylene or polypropylene containers also work, especially where corrosion or breakage may cause trouble. Back in graduate school, I learned never to trust a makeshift bottle—factory-sealed containers from certified suppliers reduce headaches later on.
Mislabeling kills efficiency and invites accidents. I once saved hours in a shared storeroom just by adding durable labels with the compound name, hazard codes, and an emergency contact number. Digital inventories work too, but nothing beats checking physical labels for real-time awareness.
Proper disposal starts on day one, not at the last minute. I’ve seen what happens when old chemicals build up in forgotten cabinets—it becomes a hazard for the next person. Always set up a clear disposal procedure, coordinated with environmental health and safety specialists. Segregate unwanted material in sturdy, clearly marked containers until professional disposal. Regular reviews of chemical stock help catch outdated or unneeded supplies before they pile up.
Safe chemical storage doesn’t require special talent, but demands consistency. Routines save lives and property, and while rules might sound repetitive, following them keeps labs open and people healthy. Treating storage as a daily discipline sets a trustworthy foundation for any work involving potentially hazardous substances.
1-(2-Chloroethyl)Piperidinium Chloride isn’t something you find in the kitchen. This compound, known for its role in laboratory research and chemical synthesis, acts as an alkylating agent. If you’ve ever worked with chemicals that react strongly with skin or mucous membranes, you’ll see similarities here. Its ability to interfere at the cellular level makes it potent for experiments but also means risks climb fast without smart handling.
Coming from a background in chemical labs, gloves serve as the dividing line between a safe shift and a costly mistake. Skin contact with 1-(2-Chloroethyl)Piperidinium Chloride can burn or blister. I’ve seen latex gloves break down from certain solvents, but nitrile tends to endure longer sessions. Splash goggles or even a face shield provide a barrier from accidental sprays—which always seem to happen if you rush or lose focus.
Safety showers and eyewash stations in the lab aren’t just window dressing. Take it from those days I saw someone miss a spot during a rinse—the difference five seconds can make stays with you. If you work with such chemicals, knowing your closest exit, eyewash station, and shower could be more important than knowing the full reaction mechanism.
Chemical vapors don’t give warnings before they cause trouble. This compound can release fumes that irritate the nose, throat, and lungs. Good ventilation isn’t a luxury—it keeps regular work possible. If your workspace gets stuffy, or if you notice a sharp, odd smell, you’re better off stepping back and checking the ventilation. I watched a friend ignore that, only to end up with headaches and coughing for hours. Fume hoods work; don’t bypass them to save time.
Respirators might sound intimidating or overkill to newcomers, but with these chemicals, precaution lowers the odds of an emergency room visit. Fit checks become second nature after awhile, and as someone who once skipped that step, I can vouch for the lingering taste of chemicals in your mouth as a clear warning sign.
These chemicals want cool, dry, and secure containment. Leaving even small bottles out in the open means risking someone's health or enabling accidental mixing. At one point, I watched glassware shatter because someone set incompatible reagents together on a warm shelf. Locked cabinets and clear labeling save more than just time; they save headaches and hospital bills.
Segregation cuts risks down. Keep 1-(2-Chloroethyl)Piperidinium Chloride apart from acids, bases, and reducing agents. Mixing mistakes lead to heat or toxic byproducts. I’ve cleaned up my share of messes, and the smell alone after a bad mix-up burned for hours.
Spills happen, usually after hours or on a Friday. If you find yourself with a puddle, don’t grab paper towels or run for water right away. Absorbent pads designed for chemicals, followed by secure disposal in clearly marked containers, prevent spreads and protect sanitation workers. My early days saw me wanting to “just mop it up” before a mentor set me straight on contamination risks. That tough talk kept me from bringing hazards home on my shoes.
Before tossing out any leftovers, check your facility’s hazardous waste protocols. Waste streams matter. Proper labeling and double-bagging wastes cut contamination. Not every city handles these scraps the same way—some need special transport, so make those calls before end-of-day.
Training new team members demands seriousness. Walk people through proper PPE, labeling practice, and emergency drills. Mistakes happen less often when everyone owns their role. Document any exposures: the more you track, the more you find patterns worth fixing before real harm.
Being cautious with 1-(2-Chloroethyl)Piperidinium Chloride shouldn’t come from a place of fear, but from respect for its power—and a commitment to keeping every team member healthy and heading home in one piece.
People who work with chemicals know the hassle of tracking down simple answers about a compound. Sometimes information about older or less common chemicals doesn’t show up easily online, or the details have gaps. 1-(2-Chloroethyl)Piperidinium Chloride is one of those substances. It pops up in organics labs, chemical research, and even in some medicine-adjacent queries. But the most basic question—does it dissolve in water, and if so, how easily—doesn’t always get an easy answer.
Anyone who’s measured out a powder in a lab, only to watch it clump at the bottom of a beaker, knows how frustrating poor solubility can be. The work slows down, reactions change, sometimes safety suffers. Some researchers spend hours on a project that grinds to a halt just because the starting material refused to dissolve.
1-(2-Chloroethyl)Piperidinium Chloride, with a structure familiar to those who studied alkylating agents, provides a good example of this irritation. The molecule sports a piperidinium ring with a 2-chloroethyl group and a chloride ion for balance. Salts like this often behave well in water, but nothing beats checking. Old lab books from school say, “as a chloride salt, it should go into water,” but real-life lab work doesn’t always match textbook predictions.
Chemical reference texts, such as the Merck Index and Sigma-Aldrich catalogs, give insight. Chloride salts tend to dissolve, especially those lacking bulky hydrocarbon groups. A piperidinium core with a chloroethyl side chain does carry some hydrophobic lean. Still, the charged nature of the salt tilts the balance toward water solubility. Most working chemists I know—myself included—would simply tip the powder into water and stir, expecting it to clear. For 1-(2-Chloroethyl)Piperidinium Chloride, reports indicate it dissolves reasonably well in water at standard concentrations used in organic syntheses.
Stronger organic solvents like ethanol or acetone don’t always play as nicely with salts, but methanol or a touch of dimethyl sulfoxide (DMSO) often pulls them in if needed. A few researchers mention that while you can get it into water, you may need gentle heat to speed things up when you want higher concentrations, especially in colder labs or with older samples.
Solubility isn’t just about mixing and stirring. It impacts everything from reaction efficiency to safety during scale-up. Knowing what solvents will work saves time and money—and can prevent accidents. I’ve seen more than a few frustrated graduate students reach for a more dangerous solvent out of impatience with slow dissolution. Clear, direct information in safety data sheets or supplier catalogs reduces that risk.
Data transparency raises trust, both in everyday chemistry work and in broader research. When solubility numbers show up in credible sources and include temperature ranges and maximum concentrations, teams make better choices. Sharing personal lab results—or updating Material Safety Data Sheets with real-life handling notes—means fewer headaches for everyone down the line.
People in laboratory and industrial settings need facts more than formality. If you have any hands-on experience with 1-(2-Chloroethyl)Piperidinium Chloride dissolving in water or solvents, publish your results or add comments where future users can find them. Keeping this type of information public not only boosts day-to-day laboratory work, but it also guides students, research newcomers, and anybody who stumbles onto an old bottle in storage. Reliable, shared, and up-to-date details set everyone up for smarter, safer, and faster work.

| Names | |
| Preferred IUPAC name | 1-(2-chloroethyl)piperidin-1-ium chloride |
| Other names |
ACE Quaternary nitrogen mustard |
| Pronunciation | /ˈwʌn tuː ˈklɔːrəʊˌɛθɪl paɪˈpɛrɪdɪniəm ˈklɔːraɪd/ |
| Identifiers | |
| CAS Number | 2386-54-1 |
| 3D model (JSmol) | `3D structure;JSmol;C1CN(CCN1)CCCl.Cl` |
| Beilstein Reference | 1711101 |
| ChEBI | CHEBI:131189 |
| ChEMBL | CHEMBL2105969 |
| ChemSpider | 18693 |
| DrugBank | DB08371 |
| ECHA InfoCard | 03e5e90b-ecf6-4b3a-b6bc-851ff20e6dfa |
| EC Number | 221-107-5 |
| Gmelin Reference | 85987 |
| KEGG | C19102 |
| MeSH | D015281 |
| PubChem CID | 156087 |
| RTECS number | TL8750000 |
| UNII | 30H5DJ67RQ |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C7H16Cl2N |
| Molar mass | 211.12 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | D: 1.099 g/cm3 |
| Solubility in water | soluble |
| log P | -2.3 |
| Vapor pressure | 0.0000133 mmHg at 25 °C |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | -0.5 |
| Magnetic susceptibility (χ) | -66.7e-6 cm³/mol |
| Viscosity | Viscosity: 51 cP (20°C) |
| Dipole moment | 6.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 296.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -251.6 kJ/mol |
| Pharmacology | |
| ATC code | N07AB02 |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS06,GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Lethal dose or concentration | LD50 (rat, oral): 75 mg/kg |
| LD50 (median dose) | 92 mg/kg (intraperitoneal, mouse) |
| NIOSH | SN4575000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| REL (Recommended) | 0.5 mg/m^3 |
| Related compounds | |
| Related compounds |
Piperidine N-Ethylpiperidine Chloroethylamine Bis(2-chloroethyl)amine Piperidinium chloride 1-(2-Chloroethyl)piperidine Quaternary ammonium compounds Nitrogen mustards |