My years trailing the footprints of synthetic chemistry make one thing clear about the story of 1-(2,5-dichloro-3-thienyl)ethan-1-one: the journey mirrors how targeted modifications pushed bench chemistry from broad discovery to specialty design. Early developments in thienyl ketones ramped up in the centermost years of the 20th century, back when aromatic sulfur compounds promised plenty but chemists still learned the ropes with organosulfur methods. By the 1970s, systematic halogenation methods let researchers get their hands dirty, tweaking the thienyl ring with precision—especially when they folded in chlorine atoms. Timing was everything: regulations hadn’t yet clamped down on halogenated intermediates, so the knowledge leap stayed brisk. With analytical instrumentation coming along, chemists tracked purity, monitored reaction pathways, and started to catalog the properties that would later set 1-(2,5-dichloro-3-thienyl)ethan-1-one apart as a workhorse in fine chemical synthesis.
1-(2,5-Dichloro-3-thienyl)ethan-1-one stands out in the catalog of heterocyclic ketones for the potent twist its dichloro substitution brings to the thienyl system. Its every molecule hints at how thoughtful design shapes performance; the addition of chlorine to the 2 and 5 positions tweaks electronic effects, nudging reactivity and selectivity in ways that plain thienyl analogs never could. In the hands of practiced chemists, the compound’s robust structure and pronounced electron-withdrawing effect expand its reach into agricultural, pharmaceutical, and specialty material applications, forming scaffolds or intermediates that open doors impossible with simpler aromatics.
From my hands-on work, the ketone usually appears as an off-white to pale yellow solid with a distinct odor—one that hints at its underlying sulfur and halogen mix. With a molecular weight hovering around 221 g/mol and a melting point centered near 82°C, it stacks up as manageable under ordinary laboratory conditions. Its solubility in common organic solvents such as dichloromethane, chloroform, and ether streamlines purification, but straight water solubility stays low, as anyone fumbling through a separation funnel will tell you. Chemically, it holds up under mild bases, but strong acids or reducing agents can break down the ring or strip away the ketone group, which makes careful choice of synthetic partners key in downstream reactions.
Any lab worth its bench space looks at purity first. Commercial batches push above 97% purity, yet for sensitive applications, analytical checks (NMR, GC-MS, and melting point analysis) weed out tricky contaminants. Labels must state the IUPAC name, batch number, and precise mass, but regulators have tightened up—hazard pictograms linked to irritant and environmental risk remain standard. There’s no mercy for casual handling now, so every bottle sports tamper-proof sealing and hazard data sheets kept in easy reach. Shipping demands tight packaging under UN guidelines for environmentally hazardous chemicals, with no leeway for shortcuts.
Synthesis of 1-(2,5-dichloro-3-thienyl)ethan-1-one traces back to two main approaches: direct acylation and controlled halogenation. Beginning with 3-thienylacetone, selective chlorination punches up the 2 and 5 positions, relying on chlorine gas or N-chlorosuccinimide as the halogen source. Precision around temperature, catalyst choice, and solvent all set the yield and minimize byproducts. Alternatively, a Friedel–Crafts acylation drops an ethanoyl group onto 2,5-dichlorothiophene using AlCl₃ as the Lewis acid, with stoichiometric care making the difference between clean conversion and a mess of polysubstitution. Both routes carry their headaches: halogenated wastes require collection, and reactive intermediates test the patience of those on cleanup crew.
Chemists after functionalized scaffolds prize the reactive carbonyl of this ketone. Reductive amination unlocks arylamine derivatives, which find their way into pharmaceuticals or fungicides. Nucleophilic additions capitalize on the electron-deficient carbon; Grignard reagents or enolates bring bulk or chirality to the ring with surprising efficiency when conditions fall just right. Further halogenation, sulfonation, or even Suzuki coupling reactions offer a range of downstream possibilities. A tweak here or there, guided by operator skill, and the base structure evolves into an array of advanced intermediates ready to anchor new projects—one of the real joys of specialty organic synthesis.
Chemists swap out names based on setting and tradition, though the compound appears most under 1-(2,5-dichlorothiophen-3-yl)ethanone. The registry often lists it as 2,5-dichloro-3-thienyl methyl ketone, but supply chains shorten it for practicality: DCTE or DCTK. In a pinch, you’ll spot oxide tags or lab shorthand, but the best practice sticks to CAS references for safety’s sake and regulatory tracking—no one in this game wants to mistake a label and ruin a batch.
Clearing the regulatory hurdles means more than wearing gloves and a lab coat. My experience taught me to treat all halogenated thienyl derivatives with respect; contact can cause dermatitis, and vapor inhalation sometimes triggers headaches after hours in a closed room. Goggle fog and double-gloved hands became routine because splashes sting and cleanup carries risks. Fume hoods, spill protocols, and closed waste containers help keep staff safe, while proper storage—dry, dark, out of sunlight—maintains compound integrity. Staff training isn’t a luxury—each operator learns to run through hazard data, spill response, and medical procedures with the seriousness the material demands.
Field experience reveals this compound’s versatility. Agrochemical teams unlock new fungicides by tuning the aromatic scaffold for disease-specific targets while keeping off-target toxicity low. In pharmaceutical development, the ketone heads into research programs studying sulfur-rich heterocycles—some exploratory drugs aiming for anti-inflammatory or antimicrobial effects got their start from a substituted thienyl backbone. Specialists working in dye chemistry pull value by exploiting the ring’s electron structure for stable, high-intensity colors, while materials scientists experiment with its electronic properties for niche semiconducting applications.
Modern R&D rides high on high-throughput screening, where derivatives of 1-(2,5-dichloro-3-thienyl)ethan-1-one pass through biological and environmental filters before ever seeing scale-up. I’ve watched teams use computational models to predict binding affinities and metabolic stability, feeding these predictions into focused syntheses. Analytical chemists measure trace levels in soil or water for environmental impact studies—continuing pressure from authorities makes that vigilance necessary, not optional. Formulation scientists bend its physical properties to fit controlled-release platforms or tailor volatility for safer, targeted use. Each new line of inquiry opens fresh challenges around reactivity, selectivity, and safe deployment, teaching each new generation a little more about what careful molecular design can deliver.
Inhalation or consumption always raises alarms with halogenated organics. Real-world data from animal studies points to moderate oral and dermal toxicity, with the main risks stacked against chronic exposure rather than single-dose incidents. The thienyl ring breaks down under metabolic stress, but chlorine substitutions slow the process, which boosts persistence in biological systems—a double-edged sword. Mutagenicity screening remains a routine task, given regulatory concerns about such halogen-bearing intermediates, and environmental tests probe the breakdown in soil and aquatic settings. Downstream chemical users push for minimized emissions and advocate for closed systems to keep exposure near zero, driven as much by cautionary tales as by precise toxicological numbers.
Looking out over the lab benches and industrial drums, the field seems set for careful, targeted use of this structure. Research into green chemistry solvents and benign-by-design methods offers hope for future synthesis and disposal, aiming to lessen environmental weight. Advances in real-time monitoring and automated process controls promise tighter operations, with less variation and waste. Intellectual property trends show growing interest in functionalized thienyl derivatives as bioactive agents and specialty materials. Across industries, the next wave of work seems set to balance value in applications with the steady drumbeat for sustainability and worker safety. That tension continues to shape how 1-(2,5-dichloro-3-thienyl)ethan-1-one will feature in tomorrow's breakthroughs and regulations.
1-(2,5-Dichloro-3-Thienyl)Ethan-1-One isn’t the sort of compound people chat about over dinner, but step into a lab, and you’ll find it working behind the scenes. Folks in chemistry circles know this molecule brings solid utility to organic synthesis. Its structure makes it attractive for building more complex molecules, since both the dichloro-thienyl group and the ketone give chemists handles to grab onto and modify in targeted ways.
Drug researchers chase new molecules all the time, searching for an edge against tough diseases. The backbone provided by the dichlorothienyl unit offers a route to synthesize new drug candidates. The presence of both sulfur and chlorine atoms in the backbone adds possibilities for medicinal modifications—some groups report improved bioactivity or better targeting when adding thienyl fragments into their pharmaceuticals. And that ketone? It’s much more than window dressing. The carbonyl group reacts easily, which drives chemists to use it in forming rings or joining new fragments, all in pursuit of inventive treatments.
Crops need defending, and that challenge motivates the creation of novel fungicides and herbicides. Certain thienyl derivatives, like this one, have found a place as intermediates in pesticide research. Plant protection scientists look for ways to slip new atoms into their products that bugs or fungi haven’t seen before. The dichlorothienyl pattern lets them play molecular chess, hoping to stay ahead of resistance. On the farm, this means better crop health and fewer chemical applications, since potent actives get the job done with smaller amounts.
Industries chasing specialty materials look for new monomers for resins and polymers, and this compound brings a unique twist. That thienyl ring stacked with chlorines turns out to boost thermal stability and resistance to corrosion. In an electronics workshop, technicians want insulators and coatings that hold up under heat and don’t break down in harsh conditions. Using such a compound in a polymer backbone brings another layer of protection—great for circuit boards or solar cells where heat and chemical stress chew up weaker materials.
Researchers love compounds like this for screening new transformations. Want to test how a brand-new catalyst holds up? This molecule’s functional groups react without a lot of fuss or unexpected detours, giving quick feedback about whether a reaction works. Students in the lab pick this compound as a model for learning techniques like Friedel–Crafts acylation or halogen manipulation, since it reliably produces clear, trackable results on the bench.
To get more out of compounds like 1-(2,5-Dichloro-3-Thienyl)Ethan-1-One, teams should focus on greener routes for synthesis and cleanup. Current methods produce some waste, and safer, lower-impact solvents cut both risk and cost over time. Sharing know-how between pharmaceutical, materials, and agrochemical researchers often sparks ideas—one group’s waste product can turn into another’s building block. By encouraging communication, science gets further with the resources in hand. Training the next wave of chemists to understand environmental responsibility alongside molecular design means new molecules don’t just work better—they leave the world a bit cleaner, too.
Every time I pick up a bottle labeled with a purity percentage, I remember my days running late-night experiments in a dusty undergraduate chemistry lab. Even small swings in purity could mean the difference between a clean result or a ruined afternoon. Purity numbers aren’t just technical jargon—they signal reliability. If a chemical claims to be 99.9% pure, I expect only the barest trace of anything else inside. Those tiny traces shape reactions, influence research data, and impact safety.
Pharmaceutical companies count on strict purity levels because even tiniest contaminants can spark allergic reactions or change how a drug works. In food processing, off-target ingredients pose safety concerns and legal risks. Environmental labs need accurate measurements to track pollution. Purity becomes more than an academic concern—it keeps people safe, research honest, and industries compliant.
Most bottles hide a backstory. Purity ratings only tell part of the truth. Where did the sample come from? How careful was the supplier during production? Testing isn’t the same across suppliers. I once compared two “identical” solvents: both promised 99.5% on the label, but only one left my glassware streak-free after cleaning, hinting at hidden leftovers. Analytical techniques—HPLC, GC-MS, and titration—each reveal different pieces of the impurity puzzle. Sometimes, dangerous impurities slip past the standard tests.
The best suppliers offer more than a tidy number. Certificates of analysis should list detection methods, batch numbers, dates, and even identification of trace contaminants. Companies with a long track record and strong auditing outshine cheaper, less-documented sources. I’ve seen friends burn through grant money because “budget” chemicals led to fouled data and repeat experiments. A small investment in quality paid off in the long run.
Ask for documentation—don’t treat labels as gospel. Check if the certificate aligns with reputable international standards, like those from USP or ASTM. Ask questions about manufacturing controls and analytical instruments. Watch out for vague or missing details. Even with a trustworthy supplier, I run spot checks. Techniques like simple melting point comparisons, or TLC for organic products, reveal a surprising amount for little effort.
Relying on just a number isn’t enough. As a user, dig into the details before buying or using a new product. Push suppliers for complete data—not just numbers, but explanations of how those numbers came to be. For research teams and purchasing departments, a solid relationship with a supplier boosts accountability. Policy makers can enforce clearer standards, closing loopholes where shady actors can cut corners.
Chemical purity decisions ripple through entire industries and impact public health. Evidence, not just reputation, builds trust. For those handling chemicals day in and day out, demanding transparency and doing the extra verification sets the foundation for safe, reliable results.
Anyone who’s worked in a pharmacy, restaurant kitchen, or even just tried to keep leftovers fresh at home knows the value of smart storage. It goes a long way in making sure what we’re keeping remains as safe and effective as possible. Trust in a product—food or medicine—is rooted in how it’s handled along the way. Once it leaves a manufacturer, conditions can make or break the contents down the line, and I’ve seen the effects of poor storage play out in real time.
Refrigerators aren’t just for leftovers. Most vaccines need a precise temperature range—typically 2–8°C—to stay viable. Forget to close the cooler in the back room, and you could lose a whole batch. Dairy farmers, grocers, and hospitals lean on standards set by folks like the CDC and FDA to keep perishables from turning dangerous. Humidity proves just as disruptive as temperature shifts; grains stored with too much moisture attract mold and insects, leading to waste and, sometimes, costly product recalls.
Light can degrade certain products at a surprising rate. I think about olive oil sitting in clear bottles near a sunny kitchen window—smells rancid after just a few weeks. Pharmacy shelves often carry dark glass vials for the same reason; light exposure messes with chemical stability. Oxygen, too, sneaks in where lids don’t fit tight. Coffee goes stale fast left open on the counter; medicines lose potency; vitamins clump and break down. Airtight packaging is a simple detail, but its impact lasts from warehouse to home.
Sometimes it helps to remember what’s really at stake. Unreliable storage conditions can mean serious health risks for patients and consumers. In countries where power isn’t consistent, vaccines spoil before they get to clinics, leading to outbreaks that could’ve been prevented. In my kitchen, wilted greens or stale crackers might just mean a wasted snack, but on a larger scale—restaurants or grocery chains—it’s a hit to reputation and safety.
Solutions start with training. Employees who know what temperature and humidity their stock needs won’t miss signs of trouble. I’ve learned that regular checks—using real thermometers, not just trusting gauges on fridges—catch problems early. Data loggers have come a long way, sending alerts straight to phones before trouble spirals.
Clear labels work, too. Marking “store below 25°C” or “keep refrigerated” right on the box removes guesswork. Smaller businesses can borrow practices from bigger operations—like using cool storage boxes for food donations or mobile vaccine fridges for clinics in remote areas. For chemicals and pharmaceuticals, investing in climate-controlled storage isn’t glamorous, but it pays off every time a batch passes inspection.
Strong storage routines reflect on everyone involved. Good habits mean fewer recalls, less waste, and ultimately, healthier customers. From what I’ve seen, keeping things cool, out of the sun, and tightly sealed amounts to more than just following rules—it protects lives, businesses, and trust.
People see a chemical name and sometimes start to worry. I get it. I spent years working in labs, storage rooms, and even a few loading docks. You open a drum, or a bag shows up on your pallet, and you pause: do I need gloves, a mask, or a safety shower nearby? You don’t want to be paranoid, but you also don’t want to be casual about safety.
Here’s what I learned: not every alarming name equals disaster, and the best way to judge real risk is by looking at facts, not just fear or habit. Take sodium hydroxide: it eats through flesh if you spill it on your skin. On the other hand, sodium chloride is just table salt. Both have "sodium" in their name. Judging danger without context, or by stories alone, leads to mistakes. Trusting the real details matters.
I grab the SDS, because it’s basically a chemical's personal history. It tells me about toxicity, flammability, reactivity, and environmental risks. It’s not just about reading, though; you need to understand those hazard symbols and numbers. A simple triangle or exclamation point in the wrong spot means take it seriously, not stuff it on a shelf next to the snacks.
Take benzene, for example. Decades ago, folks didn’t respect it in factories. Now, everyone knows exposure links directly to cancer. On the flip side, many solvents sold in the hardware store, like isopropyl alcohol, demand some respect but don’t create long-term harm if you use them wisely. The details make all the difference. Shortcuts in reading up can end up costing you later.
Using the right gear is more than a box-check. Wearing nitrile gloves, eye protection, and sometimes a lab coat became second nature for me, and that habit came from seeing accidents up close. A slip-up with hydrochloric acid led to nasty burns on a coworker’s arm, but quick thinking and good training limited the damage. No one forgets that kind of lesson. You stop rolling your eyes at goggles. You start asking questions about what’s in the bottle and double-checking before mixing stuff together.
Some chemicals don’t bite back right away. They build up over time—a small spill here, a sniff there. Lead and mercury work like that; tiny exposures still add up. That’s why people doing plumbing or lab work need to know the long-term risks, not just whether a vapor stings their nose.
Solid research means combining the SDS with guidelines from trusted sources like the CDC, OSHA, and the EPA. These organizations run studies, track outcomes, and update rules when new discoveries emerge. I’ve run into workers who claim, “We’ve handled this stuff for years, never got sick,” but history teaches us that hidden dangers don’t show up immediately.
Companies have a role. Good employers offer training, label chemicals clearly, and maintain safe storage. I’ve seen “mystery bottles” in break rooms—left over from some old experiment or job. Those scare me more than anything, because unknowns are always the hardest risks to manage. Proper identification, even for routine stuff, keeps everyone safer.
Instead of guessing, always look for reliable information. Ask experts if you aren’t certain. Don’t treat chemicals as villainous or harmless based on rumors, packaging, or old habits. A culture of safety doesn’t just come from rules—it comes from watching out for each other, staying curious, and treating unknowns with respect. There’s nothing heroic about skipping precautions, and nothing embarrassing about double-checking if gloves or ventilation are needed. In my experience, asking that extra question saves trouble every single time.
Most folks don’t spend time thinking about molecular weight or how a compound’s atoms connect. Usually, those numbers and diagrams seem meant for chemists or pharmacists hunched over textbooks. Yet, these details help shape everyday products—from food additives to over-the-counter medicine.
When I walk into a pharmacy, I see rows of painkillers, allergy pills, and syrup bottles. Flip each box over, and the label lists more than just the drug name. You’ll find exact dosages, specific chemical names, and, if you look up the ingredient insert, references to molecular weights and structural diagrams. This isn’t just showy jargon—it’s a road map for safety. The molecular weight of a compound tells pharmacists how to measure doses accurately. Too much or too little can mean the difference between relief or risk. For instance, Paracetamol carries a molecular weight of 151.16 g/mol, and pharmacists rely on this to avoid accidental overdose, especially in children.
Structure goes hand in hand with weight. A molecule's shape controls what it will do inside your body. For example, ibuprofen, with its distinct propionic acid group, binds to pain and inflammation receptors. This targeting only works because of how atoms arrange themselves—like the lock-and-key model taught in high school biology. Without clear structural blueprints, there’s no guarantee a generic pill works the same as the name-brand one sitting next to it.
Food manufacturers also lean on these details. Take aspartame, an artificial sweetener. Its molecular weight, 294.30 g/mol, influences how much ends up in a soda can. The structure of aspartame matters just as much: slight changes, like shifting a single atom, might carry health risks or strip away sweetness. Food safety regulators check every batch for purity by confirming both molecular weight and structure. The wrong form means off-flavors or potential allergens sneaking through.
Chemical data doesn’t live on a dusty shelf. It provides the transparency consumers expect from companies and regulators. The FDA and similar agencies don’t just skim the surface; they require pharmaceutical makers to submit molecular weights and validated diagrams for every batch. Having been in several labs, I remember the knots in my stomach if results didn’t line up with reference standards. One off-kilter weight or structural anomaly forced us to review every step of our process.
Problems show up when transparency slips. Contaminated cough syrup, misbranded food, and recalled supplements often trace back to mixing up molecules or using the wrong structural form. Accurate information keeps the supply chain honest—from ingredient supplier to final product.
Access to verified molecular data matters beyond big labs. Public chemical databases and QR codes on packaging help students, researchers, and watchdog groups check facts in seconds. Comprehensive ingredient listings support people with allergies who need specifics down to the molecule.
Companies should make it easy to find these details and explain the relevance in plain language. More scientists engaging with consumers, more teaching in schools about reading chemical labels, and stronger government accountability all help prevent mistakes. It’s not just a matter of numbers; it’s a matter of public trust.
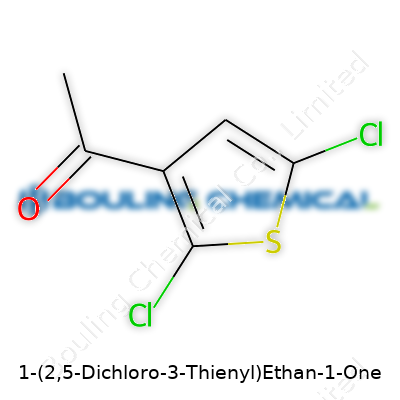
| Names | |
| Preferred IUPAC name | 1-(2,5-dichlorothiophen-3-yl)ethan-1-one |
| Other names |
NSC 405209 1-(2,5-Dichlorothiophen-3-yl)ethan-1-one |
| Pronunciation | /waɪn tuː faɪv daɪˈklɔːroʊ θaɪˈiːnɪl ˈɛθən wʌn oʊn/ |
| Identifiers | |
| CAS Number | 67668-16-8 |
| 3D model (JSmol) | `3Dmol.js:load("data:model/xyz;base64,MQogICAgMi41MCAtMC4xOCAwLjAwIENsCjEuMTAgLTAuNjAgMC4wMCBDbAoyLjM4IDAuOTUgMC4wMCBUaAoxLjI1IDEuODMgMC4wMCBBcgw4MC0xLjAwIDAuMDAgQwo=")` |
| Beilstein Reference | 1364775 |
| ChEBI | CHEBI:86345 |
| ChEMBL | CHEMBL255205 |
| ChemSpider | 230973 |
| DrugBank | DB07936 |
| ECHA InfoCard | 03b502e5-1741-4f71-be29-600e400eeec3 |
| EC Number | 67553-50-4 |
| Gmelin Reference | 14372 |
| KEGG | C19277 |
| MeSH | D016648 |
| PubChem CID | 87316843 |
| RTECS number | KH8575000 |
| UNII | BT94WZ81I6 |
| UN number | NA1993 |
| CompTox Dashboard (EPA) | DTXSID7032508 |
| Properties | |
| Chemical formula | C6H4Cl2OS |
| Molar mass | 221.10 g/mol |
| Appearance | Light yellow to yellow solid |
| Odor | Odorless |
| Density | 1.49 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.95 |
| Vapor pressure | 4.2E-4 mmHg at 25 °C |
| Acidity (pKa) | pKa = 19.74 |
| Basicity (pKb) | 14.46 |
| Magnetic susceptibility (χ) | -0.00042 |
| Refractive index (nD) | 1.622 |
| Viscosity | 0.945 cP (25°C) |
| Dipole moment | 2.93 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 271.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -74.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -877.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CM23 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P264, P271, P280, P301+P312, P305+P351+P338, P330, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 109 °C |
| Autoignition temperature | 240 °C |
| Lethal dose or concentration | LD50 Oral Rat 500 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | KM8 |
| REL (Recommended) | 0.05 ppm |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
1-(2,5-Dichlorophenyl)ethan-1-one 1-(2,3-Dichlorothienyl)ethan-1-one 1-(2,5-Dibromothienyl)ethan-1-one 1-(2,5-Dichloro-3-pyridyl)ethan-1-one 1-(2,5-Dichloro-4-thienyl)ethan-1-one |