Looking back, the story of 1,2,4,5-Tetramethyl-1H-Imidazole tracks the wider growth of heterocyclic chemistry during the surge of organic synthesis in the twentieth century. Imidazoles, a family known for their ring-shaped backbone, found traction not only in the labs of academia but in the practical world of pharmaceuticals and industry. As researchers pursued more specialized derivatives, the attachment of four methyl groups to the imidazole core led to the isolation and synthesis of this compound. Such modifications often came about as chemists searched for molecules with better stability, altered reactivity, and new potential uses. Decades of incremental progress—from the first imidazole synthesis in the 1850s, to the fine-tuned methodologies using more selective alkylation protocols—built the foundation behind the wide use and study of this molecule.
Among organic reagents, this compound stands out for the way methyl substitution affects electron density and steric factors on the imidazole ring. That means chemists often select it to influence reaction rates or modulate catalytic activities. It frequently appears as a white to off-white powder, easy to recognize for those who have handled crystalline heterocycles in the lab. Manufacturers gear up for customers working at all scales—small vials for academic experiments, or larger drums for industry. Most bottles ship with clear, no-nonsense labeling, displaying the chemical structure with its tight five-membered ring and four methyl groups dotting the skeleton. Whether for research, catalyst design, or reference use, users reach for it because it delivers a combination of steric shielding and baseline nucleophilicity common to methylated heterocycles.
1,2,4,5-Tetramethyl-1H-Imidazole bears a melting point that sits well above room temperature, hinting at strong intermolecular forces thanks to the stacked methyl groups. It dissolves in most organic solvents, especially those without strong hydrogen-bonding capabilities. Where solubility matters—like in analytical work—such properties let researchers blend it into their choice of medium without fuss. As for its chemical character, those four methyls push electron density into the imidazole ring, making it a tough competitor to other, less protected imidazoles in certain reactions. This extra bulk and electron-donating power affect basicity and nucleophilicity, so folks tweaking reaction conditions need to remember how structure tailors function. The compound stays stable under common storage conditions if protected from prolonged exposure to light and moisture, which can degrade purity over time.
Day-to-day lab work means caring about lot numbers, purity grades, and reliable documentation. Suppliers answer with detailed certificates of analysis, breaking down not just assay purity—often upwards of 98%—but also moisture content, residual solvents, and heavy metals. For those working in regulated industries, this attention to detail supports traceability and helps cut down on batch-to-batch surprises. Labels echo these priorities, handing over molecular weight (136.21 g/mol), CAS registry number (6819-73-4), and hazard information straight from regulatory databases like GHS (Globally Harmonized System). Nobody wants surprises when opening a fresh supply, so clarity in technical documentation matters as much as the transparency of the crystals inside the flask.
Synthesis usually starts with a substituted glyoxal and methylamine or similar building blocks. Early procedures often used acidic or basic conditions to cyclize precursors, sometimes followed by methylating agents such as methyl iodide. As chemical safety and atom economy became bigger talking points, more selective agents—sometimes greener, sometimes just less hazardous—entered the scene. In flow chemistry platforms, raw materials flow through heated columns in sequence, often avoiding the need for laborious workups. Solid-phase techniques, now in vogue for some specialty imidazoles, occasionally allow for easy purification by filtration rather than endless extraction steps. Each variation aims for fewer byproducts and higher yields, helping keep costs under control and minimizing environmental load from solvent waste and toxic reagents.
Those extra methyl groups might sound like static baggage, but they shape nearly everything this compound does. Steric hindrance blocks some reaction sites, making electrophilic aromatic substitution slower compared to plain imidazole. On the flip side, methyl groups raise electron density, which can change how the ring coordinates with metals or acts as a base. Chemists have leveraged this to create catalysts with more selectivity in cross-coupling reactions, or as a precursor in the build-out of ionic liquids—materials that have been grabbing attention in green chemistry. N-alkylation remains possible, but with careful selection of routes, as over-alkylation can ruin yields. Oxidative routes open the way to N-oxides, while selective demethylation remains tough but not unimaginable for those committed to ring manipulation.
Over the years, this compound picked up a handful of aliases: 1,2,4,5-tetramethylimidazole, TMH-imidazole, and some suppliers prefer its systematic IUPAC name. In industry and catalogs, shorthand dominates—abbreviations like TMI pop up regularly in method sections of research articles or technical datasheets. A few trade names floating around reflect the supplier’s branding, but all boil down to the same five-membered ring. This muddle of names highlights a broader truth in chemical research, where clear communication between disciplines means double-checking nomenclature—even for such a well-established compound.
From hands-on experience, nobody in a lab or plant wants to take shortcuts with chemicals. Most labels flag this compound as a mild irritant to skin and mucous membranes but place it outside the most acute hazard categories. Personal protective equipment—nitrile gloves, eye protection, well-ventilated hoods—ensures minimal contact. Disposal, too, follows established best practices: collect any spent material in solvent waste streams that will head for licensed incineration or chemical neutralization. Spills clean up quickly using absorbent pads and soap, but a well-drafted standard operating procedure leaves no guesswork when things go wrong. For bulk handlers, regular refresher training and spill kits stationed in reach add a solid layer of protection.
Chemists rarely settle for molecules that just fill a shelf; function drives adoption. This compound shows up often in organic synthesis, where its methyl groups help control reaction environments and product selectivity. Research teams crafting coordination complexes appreciate how it alters ligand fields around metal ions. In catalysis, those methyls not only tune reactivity but help prevent undesired polymerization or side reactions. The search for new battery electrolytes and ionic liquids brought imidazole derivatives into the spotlight, and this variant has earned a reputation for boosting physical properties like thermal stability and ionic conductivity. Pharmaceutical developers keep an eye on it, even though few direct drug applications have surfaced. In every case, users want measurable impact and often probe new ways to deploy this structure in high-value syntheses or functional materials.
Innovation needs molecules that let researchers ask tough questions—how do changes in structure shift reactivity or selectivity, and what routes to novel functionality exist? Studies around tetramethyl-imidazole keep answering these questions, especially where academics tackle reaction mechanisms or search for more sustainable synthetic pathways. Academic and industrial labs both probe its potential as a ligand in transition metal chemistry, sometimes to fine-tune activity in asymmetric catalysis. Materials scientists have tested its influence on composite and polymer behavior, especially when thermal and mechanical performance matters most. Many published papers use high-level spectroscopy and computational modeling to map out how methylation tweaks electronic behavior, a key asset in pushing forward next-gen materials and organic electronics.
Lab safety teams and regulatory bodies encourage careful tracking of toxicity for every new and established chemical. Data for 1,2,4,5-Tetramethyl-1H-Imidazole point mostly to low acute toxicity—rodent tests report moderate LD50 values—but complete datasets remain thin. Long-term effects or environmental fate still warrant closer study. Anyone working with this compound in vivo or near environmental waters keeps sample sizes tiny and containment tight while fresh research sorts out chronic risk. The trend toward more transparent reporting has drawn toxicologists to investigate metabolic breakdown and look for any persistent organic pollutant characteristics. Given broad chemical industry scrutiny, I expect continuous updates as new animal and in vitro data roll out.
For all that’s known about 1,2,4,5-Tetramethyl-1H-Imidazole, the horizon stretches wide. My time in the lab showed me how a handful of molecular tweaks can open whole new chapters, and industry often seizes on these changes to invent the next big material or process. Advances in green chemistry push developers to revisit classic scaffolds, searching for variants that cut waste and cost. As fields like catalysis, organic electronics, and specialty polymers climb, demand for such tailored heterocycles grows. Teams hunting new energy storage materials scan for imidazole derivatives that balance thermal endurance with ionic mobility, aiming to build safer, longer-lasting batteries. In the academic world, the molecular scaffold stands as a tried-and-true test-bed for chemical theory, mechanism mapping, and as a launching pad for cross-disciplinary leaps. The more sustainable, site-specific, and information-rich our methods become, the more room there is to rethink even familiar molecules like this one.
1,2,4,5-Tetramethyl-1H-imidazole catches attention with its compact yet unique structure. In chemistry, some molecules come with reputations that outpace their size, and this one fits the bill. The imidazole core forms a five-membered ring containing two nitrogen atoms at positions 1 and 3, and carbon atoms filling up the rest. Here, the “tetramethyl” part hints at four methyl groups attached to the ring, specifically on carbons 1, 2, 4, and 5. These methyl groups bulk up the molecule, shifting how it behaves in real-world applications.
In my lab experience, chemistry draws a clear line between structure and performance. Tossing those methyl groups onto the imidazole ring isn’t just a cosmetic tweak. Each addition tweaks the shape and charge distribution, changing how this molecule dissolves, reacts, and even how it interacts with living systems. Methylation often jacks up hydrophobicity, steering 1,2,4,5-tetramethylimidazole away from water and coaxing it into organic solvents. You can pour it into nonpolar liquids and expect better mixing and more predictable behavior than with plain imidazole, and this opens doors to roles in organic synthesis, advanced materials, and even pharmaceutical research.
Looking at the nuts and bolts, this molecule displays the formula C7H12N2. The five-membered imidazole ring—two nitrogens at the 1 and 3 spots—gets dressed up with a methyl group on both those nitrogens and the carbon atoms at positions 2 and 5. This arrangement feels crowded, forcing the ring’s electrons into configurations that can push reactivity in new directions. In synthetic work, researchers pick molecules like this for their stability and their ability to shuttle electrons without falling apart, important in catalysis and arena of ionic liquids.
Vital advances in battery technology, corrosion inhibitors, and molecular sensors often rest on finding the right building blocks. 1,2,4,5-Tetramethylimidazole, with its suite of methyl groups, balances stability with enough reactivity to remain useful. I’ve seen teams struggle with finding compounds that won’t degrade under heat or light. These methyl groups help anchor the molecule, keeping it from breaking down in rough environments.
Sometimes, textbooks treat compounds as abstract puzzles. In industry, the real-world traits—like how a molecule stands up to temperature swings or deals with acids—carry weight. The steric bulk from those methyl groups means fewer side reactions and more control during chemical synthesis. In the hands of a skilled chemist, this molecule offers a platform for building bigger and more complex structures—especially useful in advanced electronics and green chemistry.
For anyone experimenting with new catalysts or searching for less toxic reaction media, understanding these small shifts in structure leads to better choices and fewer failed experiments. Groups designing next-generation batteries already hunt for compounds that offer both resilience and tunable electronic profiles. By examining the full layout of 1,2,4,5-tetramethylimidazole, researchers shape future chemical processes with less waste. That means safer labs, more durable products, and a greener footprint.
People might never hear the name 1,2,4,5-Tetramethyl-1H-Imidazole outside of a lab, but this is one of those compounds that gives scientists more control and creativity in several areas. My early years in organic labs taught me to respect molecules that don’t just sit in a bottle—as soon as something can act as a building block, a catalyst, or a stabilizer, the impact grows bigger than most folks imagine.
Chemists lean on imidazoles all the time, especially those with added methyl groups like this one. Add four methyls to the imidazole ring and the molecule becomes less acidic, tougher, and more selective about what it reacts with. I have seen it step up as an organic base or catalyst, handling reactions where a little extra boost matters. In the lab, it often speeds things up, especially for the formation of bonds like those that show up in pharmaceutical research. Drug makers appreciate compounds that simplify the job of linking together complex ingredients.
It’s no secret the imidazole ring pops up in plenty of medicines. Extra methyl groups make this molecule less likely to interact in unwanted ways. Some research teams look at it as a template for antifungal or anticancer drugs. I remember reading papers that highlighted how its structure helps with SARS and even some bacterial resistance problems. By picking a molecule that plays well with strong or weak acids, drug designers shape medicines that break down at just the right pace.
One use that often gets overlooked comes from stabilization. Many industrial processes create things that need to last—think plastics, rubbers, or specialty coatings. Additives like 1,2,4,5-Tetramethyl-1H-Imidazole slow down the chemical wear and tear these materials face under heat or light. Experience teaches anyone who works in product development that shelf-life spells the gap between junk and a bestseller, so choosing the right stabilizer can protect companies from callbacks and bad reviews.
You can’t ignore the puzzle-solving part of advanced organic chemistry, either. This molecule opens pathways to other specialty compounds. The methylation pattern gives unique electronic properties, letting it act as a stepping stone for dyes, resins, or conductive materials. As labs try to shape substances for new electronics or imaging agents, having a flexible starting point like this speeds up the trial-and-error process. For small specialty companies, that can mean staying ahead in competitive markets.
One challenge with many high-performing chemicals? Some pose handling risks. It helps that methylated imidazoles like this one generally offer a better safety profile than related catalysts or bases. In regulated industries, better safety supports worker health and reduces clean-up costs. It’s not just about performance—value shows up in fewer shutdowns and lower insurance bills.
The movement toward greener chemistry has pushed manufacturers to rethink old habits. Compounds that work at lower temperatures, cause fewer side products, and handle recycling better—these earn points in today’s industry. 1,2,4,5-Tetramethyl-1H-Imidazole rides this shift because it lessens the need for harsh reagents and can survive repeated cycles in reaction setups. Each time companies cut a hazardous step, both safety and cost tracking improve. This push for sustainability pays off, not just for the environment, but for budgets and long-term business growth.
Purity represents more than a percentage on a label. High purity means lower risk of unwanted side effects, fewer failed batches, and less time lost tracing a mystery contaminant. If you’ve ever dealt with a batch that missed the mark by even a fraction of a percent, you know the pain of digging through paperwork and chasing lost hours. Even small impurities can swing results wildly in pharmaceutical or food settings. Pharmaceutical firms lose millions yearly from recalls driven by impurity issues, and the FDA tracks everything right back to source. Impurities sometimes cause reactions or toxicity, putting both companies and customers in a tough spot.
Not all impurities act the same way. In some cases, a tiny contaminant spurs unwanted chemical reactions or off-odors. At other times, it just clouds a liquid, ruining its look. Analysts notice these slip-ups quick, thanks to tighter rules and sharper science. High purity gives people confidence in what they're buying and using, and it helps techs or researchers trust their work’s foundation. In my own experience, labs often pay more for the best grade they can get, because it saves trouble later, and nobody wants their boss pointing fingers.
If you’re holding a fine powder, you know it’s a different beast compared to working with a solid block or a sticky liquid. The physical state influences how you store, ship, and use a product. Powders spill easily. Liquids can leak and evaporate. Big crystals sometimes need crushing. I’ve had jobs go sideways just because a drum of what I thought was pourable turned out solid on arrival in winter.
Physical state also tips the scales in mixing, dosing, and safety. A dust cloud in the air sometimes causes health risks. Some products catch fire or explode when they build up static as a powder. Liquid forms can mean faster reactions, but anything volatile or toxic needs ventilation and protective gear. Teams that ignore these factors risk equipment malfunction, accidents, or environmental leaks. Smart companies match production procedures and packaging methods to the product’s real-life condition — not what’s stamped in a catalog.
Manufacturers push to deliver higher purity and manage physical state, but costs weigh heavy. It’s not just about filters and machines. You still need steady raw supplies and sharp quality checks. Shortcuts show up fast when end-users run tests, or worse, face a recall. Trust evaporates in the supply chain if purity slips or state doesn’t match what’s promised.
Most large-scale buyers demand certificates and run quality audits. I’ve watched buyers reject a whole shipment if one drum felt too moist or clumped up. Sometimes, companies keep a backup supplier just to avoid being left in the lurch.
Basic fixes can make a difference. Start with honest lab testing, plus third-party verification for key goods. Run checks at different temperatures to cover all seasons. Use packaging that suits the product’s state — stronger barrels for liquids, desiccant packs for powders. Communicate results up and down the chain so no one gets surprised by a change in consistency or grade.
In the end, purity and state define the story of a product. They influence trust, price, and safety every step of the way. Companies that focus on both details at once usually see fewer headaches and keep partnerships steady, no matter the market.
Anyone who’s handled chemicals for research projects or industrial production knows storage isn’t busywork — it’s a big part of safety, reliability, and keeping material costs under control. 1,2,4,5-Tetramethyl-1H-Imidazole isn’t an exception. This organic compound looks simple, but its quirks demand attention. With experience in small- and mid-scale labs, I’ve seen what goes right and wrong when teams cut corners or misunderstand what practical storage means.
Some chemicals stay stable for decades with little more than a tight lid and a cozy cupboard. Imidazole derivatives rarely forgive sloppiness. Even minor exposures to air or moisture kick off contamination, slow decomposition, or in some cases, surprising reactions. What looks harmless can become a headache if left next to incompatible solvents or careless heat sources.
Fact: According to several MSDS datasheets and OSHA advisories, even a few degrees above room temperature raises volatility and evaporation rates. Scientists and safety managers know stories of minor leaks creating fumes and workplace complaints.
For years, I’ve made it a habit to store 1,2,4,5-Tetramethyl-1H-Imidazole in tightly sealed, amber glass bottles. Plastic drums look convenient, but strong bases or amines in the same cabinet eventually leach residues into plastics. Glass with a ground-glass stopper beats screw-cap lids under many lab conditions — less chance of vapor seepage, and easier to check for leaks.
Humidity corrupts almost every dry chemical over time. Keeping this compound away from direct atmospheric exposure protects both the product and the people using it. Desiccators or a glove box with dry, inert gas work even for benchtop projects as long as they’re well-maintained. I often add silica gel packs in storage cabinets for another layer of moisture absorption.
A cool spot, out of sunlight, does much of the heavy lifting. Heat accelerates chemical breakdown, speeds up pressure build-up, and in rare cases increases risk of fire. The labeling “store at room temperature” assumes the room isn’t an oven. I keep volatile or sensitive chemicals away from windows, radiators, and fume hood outlets — simple decisions save supplies from heat spikes.
Never underestimate physical separation for safety. 1,2,4,5-Tetramethyl-1H-Imidazole doesn’t mix well with oxidizers or acids. Even if two bottles never break, tiny splashes from one transfer can trigger contamination. Dividing shelves by chemical type and hazard makes these mistakes much less likely.
Rotating old stock forward and marking each bottle with the opening date helps avoid forgotten “mystery bottles.” Many mishaps I’ve witnessed started with a dusty, unmarked jar stashed in the back. Access logs, regular checks, and strict sign-out sheets save hours sorting chaos later.
Responsible storage comes down to clear organization, physical barriers, and a little common sense. Labs and companies that train all users — not just full-time chemists — see fewer accidents and spend less on lost goods. Nobody benefits from trusting chemical safety to luck or hope.
Many overlook the hazards in everyday chemistry. People talk about safety but sometimes skip some corners because rushing feels easier than double-checking. I’ve seen what happens when that attitude slips through the door—a spill during a routine transfer, a split glove during cleanup, or someone lowering their mask “just for a second.” One small lapse can lead to emergency showers, ER trips, or worse. These experiences leave a lasting impression that guides every move I make.
Regular nitrile gloves don’t stop every liquid. A lot of folks just grab the nearest pair, thinking anything is better than nothing, but some solvents and chemicals chew right through them. Before handling any compound, check for specific glove compatibility. Face shields pair with goggles, not as a replacement. Splash-resistant aprons, lab coats, and closed shoes aren’t just “lab fashion”—they save your skin and eyes.
Nobody wants to breathe in fumes. Proper fume hoods keep inhalation risks down, not just for volatile organics but for nearly everything that evaporates. I’ve worked in spaces where budget labs shared hoods and cut corners with fans. Routine exposure to low-level vapors can pile up, leading to headaches, allergic reactions, or even more serious harm over time. Well-maintained ventilation systems keep everyone safer, and regular checks ensure nothing clogs up or fails.
The habit of slapping on clear, permanent labels doesn’t just help the next shift. It stops mix-ups that can lead to dangerous reactions—someone adding the wrong reagent, or disposing of something incorrectly. Nothing wastes time like sorting through mystery containers hoping to avoid disaster. Full documentation creates accountability, and, based on my experience, people work more carefully when they know their steps are logged.
No one plans a spill, but they happen. Years back, I watched a flask tip over and dump a few ounces of a strong base. If the right absorbents, neutralizers, and emergency kits hadn’t been close, someone would have walked away with chemical burns or worse. Standard kits aren’t enough if they sit in a locked cabinet or stay empty. Everyone needs real training—knowing which kit fits which spill. Practice helps turn panic into a controlled response.
Storing chemicals by family keeps lurking disasters at bay. Strong acids and organic solvents parked side by side don’t mix well. Some compounds need cooling, darkness, or special ventilation. I’ve seen locked cabinets prevent accidents. Separate incompatible materials and use trays to contain leaks; a little up-front organization beats cleaning up after a minor explosion or corrosion.
Culture in labs and industries doesn’t change with fancy posters. Peer-to-peer checks, honest risk assessments, and encouraging speaking up about near-misses build real safety. Technology can help—inventory management software, environmental sensors, and automatic shutoffs exist for a reason. Most important, leadership should listen and act fast when people raise concerns. My take: nothing replaces vigilance, humility, and real support from both top-down procedures and bottom-up teamwork.
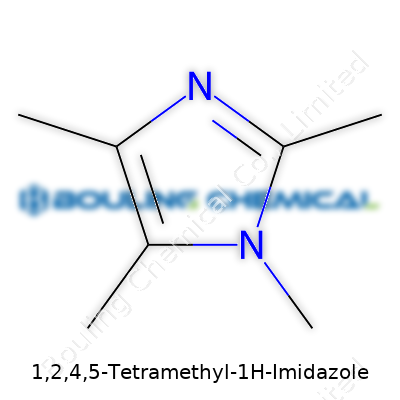
| Names | |
| Preferred IUPAC name | 1,2,4,5-Tetramethyl-1H-imidazole |
| Other names |
1H-Imidazole, 1,2,4,5-tetramethyl- 1,2,4,5-Tetramethylimidazole |
| Pronunciation | /ˌwaɪ.tuː.fɔːr.faɪv ˌtɛ.trəˈmiː.θəl wʌn eɪtʃ ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 1124-11-4 |
| Beilstein Reference | 83668 |
| ChEBI | CHEBI:36610 |
| ChEMBL | CHEMBL607241 |
| ChemSpider | 86449 |
| DrugBank | DB08441 |
| ECHA InfoCard | 03ce9846-492e-47a5-8731-125b2645b6fb |
| EC Number | EC 211-851-1 |
| Gmelin Reference | 72568 |
| KEGG | C21196 |
| MeSH | D052187 |
| PubChem CID | 69409 |
| RTECS number | NL3325000 |
| UNII | M8C20Q6A58 |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID7067602 |
| Properties | |
| Chemical formula | C7H12N2 |
| Molar mass | 124.19 g/mol |
| Appearance | White to pale yellow crystalline solid |
| Odor | amine-like |
| Density | 0.984 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 0.68 |
| Vapor pressure | 0.15 mmHg (25°C) |
| Acidity (pKa) | 13.6 |
| Basicity (pKb) | 8.28 |
| Magnetic susceptibility (χ) | -33.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.543 |
| Viscosity | 103.6 cP (25 °C) |
| Dipole moment | 2.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 213.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2713 kJ mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H225, H302, H315, H319, H336 |
| Precautionary statements | P261, P280, P304+P340, P312, P403+P233 |
| Flash point | 113°C |
| Autoignition temperature | > 415 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 820 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1,2,4,5-Tetramethyl-1H-Imidazole is 2300 mg/kg (oral, rat) |
| NIOSH | NA1250000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | `10 mg/kg` |
| IDLH (Immediate danger) | No established IDLH value. |
| Related compounds | |
| Related compounds |
Imidazole 1-Methylimidazole 2-Methylimidazole 4-Methylimidazole 1,2-Dimethylimidazole 1,4-Dimethylimidazole 1,2,4-Trimethylimidazole 1,2,5-Trimethylimidazole 1,3,4,5-Tetramethylimidazole |