Chemists in the late 20th century cast an inquisitive eye toward the versatile piperazine core, experimenting with substitutions across the aromatic ring. The synthesis of 1-(2,3-Dichlorophenyl)Piperazine marks an important step in the search for new biologically active molecules. Laboratories in Europe and North America, especially those with a focus on neuropharmacology, pursued piperazine derivatives for their impact on serotonin receptors. This particular compound carved a niche as researchers mapped out the chemical terrain of psychoactive drugs. Over decades, its structure has become a fixture in medicinal chemistry texts, not only because of its own properties but by pushing further innovation in psychopharmacology and diagnostic imaging.
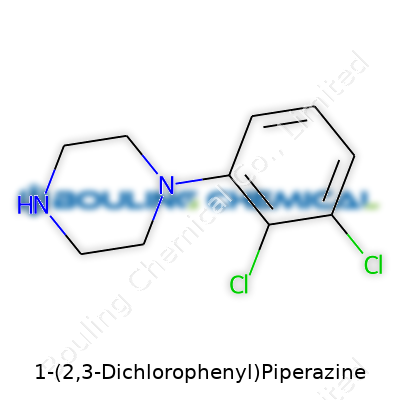
1-(2,3-Dichlorophenyl)Piperazine is best recognized within chemical catalogs as an intermediate or building block. The molecule features a piperazine ring tethered to a 2,3-dichlorinated phenyl group, making it of keen interest for those tracing the edges of serotonin modulation. Researchers looking for model ligands or synthetic precursors in the investigation of brain chemistry often reach for this compound. It's sold in both small research quantities and larger lots for custom synthesis, usually as a white to off-white crystalline powder. The real substance is found not in static identity, but in the doors it opens across neuroscience and drug discovery.
Familiar with laboratories, I've seen 1-(2,3-Dichlorophenyl)Piperazine pressed into service in many settings. Its melting point usually lands in the 80–85 °C range, which gives you a clue about handling and storage. As a solid, it dissolves sparingly in water but blends better with organic solvents like ethanol, DMSO, and acetonitrile—a common trait among chlorinated aromatic intermediates. The molecule has two chlorine atoms snug on the phenyl ring, which lend electron-withdrawing power, affecting both its reactivity and biological interactions. The combination of a basic piperazine core and electron-deficient aromatic system shapes its affinity for neurotransmitter receptors and, by extension, its roles in pharmacological research.
Purchasing from chemical suppliers, I've learned to watch for purity above 98%, which high-end research calls for. Each batch ships with a certificate of analysis and safety data, including molecular formula C10H12Cl2N2, molecular weight near 231.13 g/mol, and details on residual solvents. Labeling highlights hazard codes in line with GHS conventions, noting both irritant potential and specific handling needs. Storage instructions recommend a cool, dry place, sealed tightly to avoid degradation or moisture pick-up. Barcodes and unique lot numbers tie each jar back to its production batch, a safeguard that prevents headaches in compliance or repeat experiments.
The classic approach brings together piperazine and 2,3-dichloronitrobenzene through nucleophilic aromatic substitution, typically using a polar aprotic solvent such as dimethylformamide and a base like potassium carbonate. This reaction runs under moderate heat, driving the exchange as piperazine displaces the nitro group. Once coupled, a hydrogenation step reduces the nitro group to amine, rounding out the synthetic path. Laboratories opt for careful monitoring via TLC or HPLC to ensure conversion, then extraction, washing, and crystallization to isolate the pure compound. The route is robust and has lent itself to upscaling in custom synthesis if the market demands.
1-(2,3-Dichlorophenyl)Piperazine serves as a flexible platform for further chemical tinkering. Halogen atoms beckon for nucleophilic substitution, letting chemists install a broad set of functional groups. Using standard Suzuki or Buchwald–Hartwig couplings, it's possible to swap the chlorines for aryl or amino substituents, broadening the pool of derivatives. The piperazine ring itself holds up well under reductive amination and alkylation reactions. In my own lab experience, linking this core to larger scaffolds enabled probing into selective serotonin and dopamine receptor interactions, a practice still common in pharmacology pipelines.
Several synonyms mark this compound in scientific and industrial registries. Commercial catalogs might call it 1-(2,3-Dichlorophenyl)piperazine or, in shorthand, 2,3-DCPP. Literature from the 1990s often refers to it simply as dichlorophenylpiperazine. Less common pin names, based on IUPAC conventions, include 1-(2,3-dichlorophenyl)-piperazine. Custom synthesis outfits sometimes attach their own order codes, but the essence remains recognizable to anyone who reads the molecular structure as a kind of fingerprint.
This class of compounds, with roots in psychoactive chemistry, comes with clear guardrails. Lab work requires gloves, lab coat, and goggles—standard practice but taken seriously here due to moderate toxicity and irritant properties. I’ve followed fume hood protocols and noticed that small-scale spills demand immediate clean-up with absorbent, non-combustible materials. The GHS hazard statements warn of skin, eye, and respiratory irritation. Local waste handling codes dictate incineration of disposal materials instead of draining into wastewater pipes. For any scale above milligrams, facilities double up on training and logs to reflect evolving environmental and personal safety standards. It’s better to err on the side of caution, especially as piperazine derivatives sometimes attract regulatory scrutiny.
Serotonin research stands up as the main playground for 1-(2,3-Dichlorophenyl)Piperazine. Pharmacologists treat it as a tool compound to probe the function of 5-HT1A and 5-HT2A receptors, modeling both binding and biological outcomes such as changes in mood, perception, and behavior. Beyond pure research, the molecule pops up in the development of new antidepressants, antipsychotics, and diagnostic agents for neuroimaging. Chemists at pharmaceutical firms explore its scaffold to create analogues with improved selectivity or reduced adverse effects. Some forensic laboratories also monitor its presence in seized psychoactive mixtures, tracking the spread of research chemicals in recreational contexts. This crossover between research, development, and public health gives the molecule a real-world relevance apart from dry molecular formulas.
Academic and industry labs lean on 1-(2,3-Dichlorophenyl)Piperazine when developing small-molecule libraries for CNS drug screening. Studies in the early 2000s explored its role as an active metabolite of certain antidepressants, driving greater attention to metabolite profiling and side effect prediction. Structure–activity relationship (SAR) research benefits from the dichlorinated aromatic ring, which offers a rigid yet modifiable platform. High-throughput screening teams catalog its effects across swathes of neural receptors, mapping out off-target activities that might hint at new clinical applications. It’s not just a passive scaffold—the dichloro groups can dial down or up activity, helping drug designers pinpoint structure changes that matter. More recently, this compound has sparked interest as a lead for PET tracers, giving researchers tools to peer at live brain processes at the molecular level.
Early toxicity studies drew attention to both acute and sub-acute effects, especially at doses above those used for receptor binding studies. In rodent models, 1-(2,3-Dichlorophenyl)Piperazine produced transient changes in locomotor activity, mild agitation, and alterations in serotonin turnover. Human exposure risk appears low in controlled lab settings, but off-label or recreational use flags clear dangers, with case reports of hallucinations, agitation, or, in rare instances, seizures. Chronic toxicity data remain limited, so most safety guidelines follow the precautionary principle—minimize contact, avoid ingestion or inhalation, and treat every spill as potentially hazardous. I’ve seen questionnaire forms in institutional oversight processes specifically naming this molecule, reflecting both historical concern and ongoing vigilance among research groups.
The next decade promises new insights from this class of molecules. Medicinal chemists pursue biasing ligands that can tip serotonin signaling one way without lighting up all related pathways, and the dichlorophenylpiperazine motif keeps turning up in promising hits. With greater interest in psychiatric and neurological disorders, research dollars will likely push for improved derivatives with safer toxicity profiles. Analytical chemists develop better detection methods, both to safeguard clinical trial volunteers and clamp down on misuse. Environmentally, advances in green chemistry could reshape the way this compound is synthesized or disposed, with new catalysts and biocatalytic processes coming to the fore. The molecule stands as both a subject of direct investigation and a gateway to deeper questions about brain chemistry, therapeutic intervention, and regulatory boundaries. As neuroscience and synthetic chemistry continue to overlap, 1-(2,3-Dichlorophenyl)Piperazine sits at a busy intersection between discovery, application, and safety.
Chemicals like 1-(2,3-Dichlorophenyl)piperazine don’t get much attention outside labs and forensic circles, yet they play a curious role both in scientific research and public policy. Commonly abbreviated as 2,3-DCPP, this compound belongs to the phenylpiperazine class—structures showing up in the development of certain pharmaceuticals, as well as in recreational drug markets. For those working in clinical pharmacology or toxicology, 2,3-DCPP raises alerts for more than just its tongue-twisting name.
Scientists explore 2,3-DCPP for its interaction with serotonin receptors. Many compounds in this chemical family act on the central nervous system, which explains their appeal for studying how brain chemistry shifts in mental health disorders. In my experience, researchers reach for 2,3-DCPP when looking to compare the effects of new therapeutic candidates, especially those aimed at treating depression or anxiety. Its structure helps map out how small tweaks in a molecule can dial up or down activity in the brain. These insights push the boundaries of safer medications, offering potential shortcuts towards better antidepressants or antipsychotics.
The story changes outside controlled settings. Over the last two decades, synthetic chemists operating outside the law have used 2,3-DCPP as a building block for so-called designer drugs. It has turned up as a common impurity or metabolite in tablets sold as “ecstasy” or “legal highs.” Forensic teams have flagged it in seized pills, especially across Europe and Asia. People don’t typically set out to buy 2,3-DCPP by name, but they might end up ingesting it unknowingly because of sloppy synthesis or attempts to skirt drug laws. Public health agencies warn about toxic effects, from agitation to seizures, and a wave of hospitalizations in the mid-2010s underlined its risks. The lesson here hits hard—unregulated compounds carry unpredictable health threats.
For parents, educators, and healthcare professionals, knowing the names behind some street drugs helps keep conversations grounded in facts. I have fielded enough questions about “mystery pills” to see the gap that exists between the pace of drug innovation and public health communication. This compound’s availability online highlights the need for up-to-date drug screening tools, as emergency rooms now face rapidly changing chemical threats. Law enforcement also faces hurdles when a substance like 2,3-DCPP sits in a legal gray area, complicating prosecutions and allowing unscrupulous manufacturers to stay one step ahead.
Better communication between researchers, clinicians, and regulators makes a difference. Shared reference databases help everyone from doctors to customs officers spot new variants of these chemicals early. Testing for new substances needs investment. I have seen community outreach programs make strides by linking local health authorities with national toxicology labs. If people trust the information coming from those trained to spot harmful designer drugs, there’s a better shot at harm reduction. At the policy level, smarter scheduling laws can cut down on dangerous loopholes, though lawmakers must balance fast response with scientific evidence.
Staying ahead of the next public health threat means digging deeper into the chemistry behind emerging risks. As long as labs—legitimate or not—modify molecules to chase profits or medical breakthroughs, the role of compounds like 2,3-DCPP will keep evolving. Real progress depends on connecting the dots across scientific, medical, and legal fields, and on keeping conversations honest about what these chemicals actually do.
A lot of people come across chemical names like 1-(2,3-Dichlorophenyl)piperazine, often written as 2,3-DCPP, and wonder if they can buy it without worrying about any knock on the door. This question lands right in the gray spots of chemical law, where science meets regulation and curiosity gets tangled up with risk.
2,3-DCPP belongs to the piperazine class, related to substances that have shown up in everything from pharmaceutical research to party pills. These aren’t always the villainous substances you hear about in true crime podcasts, but some closely related that have landed on banned lists before. Piperazines have been used in the past for making new psychoactive substances, with some, like BZP or mCPP, ending up controlled because of misuse.
In the United States, laws around chemicals work like a patchwork quilt. There’s no single, clear ban on all piperazines, but the Drug Enforcement Administration (DEA) has gone after certain ones specifically because they showed up in recreational drugs. If a variant of piperazine showed up in the wrong kind of pill, the law found a way to keep it away from buyers. Directly, 2,3-DCPP barely gets name-dropped in U.S. federal schedules. It’s not sitting on the main lists of banned controlled substances.
Import law adds another wrinkle. Customs and border officials don’t play around. Even a chemical without a “Schedule I” tag can attract attention if its structure looks similar to something controlled. The Federal Analogue Act in the U.S. makes it illegal to possess or distribute chemicals that are “substantially similar” to banned substances, if the aim is human consumption. An obscure chemical used purely for lab research may seem harmless, but intent and context change the legal landscape fast.
Leave the United States and it gets even more complicated. Countries in Europe, like the UK or Germany, have swept whole families of designer drugs into wider controls. You’ll see chemicals grouped not by their specific names, but by whole classes and families. This blanket approach came along after years of “legal highs” staying one step ahead of lawmakers. I’ve seen headlines change overnight because a country’s health authorities updated their watchlist after a single incident.
People drawn to chemistry want to experiment, but legality isn’t the only consideration. Sellers often use “research chemical” labels as a loophole, but that shield is paper-thin. Customs officers, local police, or even neighborhood pharmacists can turn a simple transaction into a headache or worse. Reliable sourcing gets tricky because companies try to dodge laws by shipping from borderless, low-regulation countries. That brings customer risks – no guarantees on purity or safety, and a very real chance of direct law enforcement scrutiny.
I’ve talked to fellow researchers and hobbyists who ended up with more than they bargained for—a box on the doorstep carrying not only chemicals but legal inquiries too. Even those with clear research intentions catch the fallout when a misunderstood chemical finds its way into the wrong report or project.
Transparency stands out as the best fix. Legislators and chemical suppliers could create clearer guidelines on where research ends and recreational use begins. More databases and registries built for public, non-corporate users would help. For now, reality means doing the difficult homework: double-checking the rules in your state or country, talking to a lawyer who knows the hazy world of chemical law, and above all, letting curiosity be guided by responsibility.
People sometimes hear complicated names like 1-(2,3-Dichlorophenyl)piperazine—often shortened to 2,3-DCPP or DCPP—and think they’ve encountered some exotic, out-of-reach compound. In chemical and pharmacological research, this molecule pops up in several contexts, usually as a reference substance or an intermediate in synthesizing various drugs. DCPP draws scientific attention because of its direct effects on serotonin systems, which play a key role in mood, perception, and many bodily functions. The world of psychoactive substances is full of surprises, so any new substance demands a close look at its safety, especially the side effects it may carry.
Researchers have studied this compound for its similarities to other piperazine derivatives. People might recognize its “cousins” like mCPP, which can cause strong reactions in the nervous system. 2,3-DCPP’s relationship with serotonin means it doesn’t just pass through the body unnoticed. Scientific papers note that piperazine-type compounds have caused headaches, dizziness, anxiety, nausea, restlessness, and sometimes cardiovascular effects. Recent studies from the Journal of Psychopharmacology and reports from the European Monitoring Centre for Drugs and Drug Addiction make it clear: these effects can feel mild in some and strong in others.
Learning about a chemical by reading case reports or academic studies is one part of the story. It feels different when you talk to people who have direct experience—clinicians, researchers, and even users in experimental settings. Practitioners who have watched the piperazine class in real time often flag unpredictable side effects. Heart palpitations and chest discomfort get mentioned, and some have seen cases where people develop tremors, sweating, or agitation after exposure. Psychological effects can be just as tough to manage: paranoia and mood swings turn up in self-reports and poison center data.
The impact on the heart often prompts concern, especially with raised blood pressure and heart rate. Those dealing with a predisposition toward anxiety or a history of heart conditions face greater risk. No magic bullet exists for countering these issues; supportive care in clinical settings forms the practical backbone for handling acute problems.
A responsible approach comes from verified testing and honest communication. Laboratories already use sophisticated protocols to study new substances, flag negative effects, and share honest data. In the real world, people can reduce dangers by refusing untested compounds and seeking information before any potential exposure. In some places, such substances wind up in recreational products—users often don’t realize what’s inside. Educational campaigns can tackle the knowledge gap, especially by linking scientists, doctors, and the public.
Serious medical complications deserve quick attention. If someone displays agitation, confusion, rapid heart rate, or chest pain after suspected exposure, emergency help is crucial. Reporting adverse reactions offers data that helps shape future healthcare strategies and public safety policies.
Transparency, updated information, and scientific diligence keep communities safe. Pharmacological research continues to shed light on how 2,3-DCPP interacts with the body and mind. Open dialogue between clinicians and the people at risk leads to real solutions and lowers risk. Each story adds to our shared understanding and sharpens the decision-making that shapes health outcomes.
1-(2,3-Dichlorophenyl)Piperazine sounds like a mouthful, but it’s more than just a complicated name. Chemists use this compound in research and drug development, but don’t let its technical label fool you—this chemical comes with hazards. It can be toxic if inhaled, ingested, or absorbed through skin, and improper storage turns a research tool into a safety threat. Anyone handling it must understand how to store it safely, not only to protect their own health but also to maintain the stability and integrity of the chemical.
My own lab experience showed me that controlling the climate in your chemical storage room is non-negotiable. Warm rooms or labs with swinging humidity levels often ruin sensitive compounds. 1-(2,3-Dichlorophenyl)Piperazine prefers cool, dry, and dark places. Sunlight can break down the compound, and moisture risks starting unwanted reactions or degradation. So, keep it away from windows and sinks. Use storage cabinets that don’t face exterior walls if possible, since these spots often pick up temperature swings.
Plastic bags and thin-walled containers never cut it for chemicals like this. Sealed glass bottles work best, because even small leaks can mean trouble for both safety and research quality. I remember a case where a coworker stored an organic chemical in a plastic screw-top jar, only to discover the vapors ate away at the container over a few months. Don’t take that risk—go with glass and make sure lids fit tightly. Label everything, and don’t trust a permanent marker alone. Use chemical-resistant labels designed for lab use, because smears or faded writing just cause confusion later.
Chemicals tend to misbehave when mixed, even unintentionally. Flammable materials, oxidizers, acids, or bases should not share a shelf with 1-(2,3-Dichlorophenyl)Piperazine. One spill could start a fire or release toxic fumes. I like to keep a written record of which classes of compounds can go together and stick to those lists religiously. Cross-checking the Safety Data Sheet (SDS) every time something new goes into storage can feel tedious, but one overlooked reaction could end up on the evening news.
Leaving toxic chemicals on open shelves in a busy lab never ends well. People grab the wrong bottle, take shortcuts, and accidents happen. Always use a locked chemical cabinet for any compounds requiring extra care. Control access to avoid unauthorized use. It doesn’t take much to turn a secure lab into a health hazard. In my own work, only trained team members can unlock hazardous storage, and everyone signs their name and the date whenever they access a chemical like this one. That helps everyone stay accountable and careful.
Preparation saves lives and work. Keep spill kits nearby, not just under a sign somewhere in the building. Train everyone in their use. Gloves, safety glasses, and lab coats do a lot of the heavy lifting, but they only help if people use them correctly. Regular training keeps safety at the front of everyone’s mind. Make it part of onboarding, not an afterthought, so no one feels unsure if a bottle tips over or something leaks.
Chemicals like 1-(2,3-Dichlorophenyl)Piperazine serve important scientific purposes, but they reward careful attention. Safe storage relies on clear procedures, good habits, and real respect for what these compounds can do if ignored or mishandled. People never regret taking those extra steps, but shortcuts often bring trouble. That’s a lesson the whole scientific community keeps learning, one day at a time.
Some substances catch attention because of their presence in online forums or underground circles. 1-(2,3-Dichlorophenyl)Piperazine, or 2,3-DCPP, pops up now and then among so-called “research chemicals.” This chemical doesn’t appear anywhere on official pharmaceutical lists. It has no FDA approval, no established use in medicine, and no clinical data backing up any sort of dosage.
Folks sometimes assume that if a substance appears online, there must be a safe way to take it. That’s far from reality. Hospitals and poison control centers deal constantly with new synthetic compounds. During my years covering drug safety, I’ve heard endless stories of people harmed by unfamiliar chemicals. 2,3-DCPP acts on the central nervous system, and that sets the stage for unpredictable dangers.
Looking for “the recommended dosage” of a chemical with no approved human use is risky. I checked scientific literature, regulatory agency reports, and even underground forums: no credible organization publishes dose guidelines for 2,3-DCPP. The lack of information doesn’t mean it’s safe; it means nobody has mapped the risks.
When researchers test new compounds, they run animal studies, controlled human trials, and years of monitoring. Cutting corners with designer piperazines, people serve as unintentional guinea pigs. Reactions range from terrifying anxiety to seizures or much worse. According to the European Monitoring Centre for Drugs and Drug Addiction, certain piperazines related to 2,3-DCPP triggered hospitalizations and even deaths across Europe.
People sometimes mix up 2,3-DCPP with related substances, thinking doses align. Chemistry isn’t that simple. Small changes in chemical structure can flip a substance from “pharmacologically inactive” to “severely toxic.”
No laboratory or health agency offers dosing information for 2,3-DCPP. The World Health Organization and European drug monitoring groups flag piperazine analogues as a class with unpredictable effects. In my reporting, emergency physicians always stress how new psychoactive substances stay several steps ahead of regulation and research. Data collection doesn’t keep pace.
I’ve seen efforts to regulate these compounds increase over time, but the vast world of designer chemicals keeps growing. That means genuine experts in pharmacology and toxicology stick to one message: without quality control, dosage advice, and official studies, anything resembling “dose” remains a dangerous guess.
Education stands out as the strongest tool available. Community groups, universities, and even some harm-reduction advocates tackle the flood of misinformation. These groups highlight the dangers and urge caution. No responsible scientist or healthcare professional endorses trying experimental chemicals with no medical oversight.
Policy changes can help, including stricter regulations for undisclosed substances sold online. But enforcement alone won’t remove the risk. Curiosity goes hand in hand with access; when people search for dose suggestions, honest information about the real hazards matters more than any dubious “safe range.”
Nobody can give a responsible dosage for 1-(2,3-Dichlorophenyl)Piperazine. Searching for that answer is a signal: the chemical poses too many unknowns, and the wisest move relies on staying well clear of it.
| Names | |
| Preferred IUPAC name | 1-(2,3-dichlorophenyl)piperazine |
| Other names |
1-(2,3-Dichlorophenyl)piperazine 2,3-DCPP 2,3-Dichlorophenylpiperazine |
| Pronunciation | /ˈwʌn ˌtuː θri daɪˌklɔːrəˈfiːnəl paɪpəˌreɪziːn/ |
| Identifiers | |
| CAS Number | 63509-86-2 |
| Beilstein Reference | 1736693 |
| ChEBI | CHEBI:77773 |
| ChEMBL | CHEMBL14707 |
| ChemSpider | 141557 |
| DrugBank | DB08238 |
| ECHA InfoCard | 03b5584c-68fe-49d6-9323-1bac06b9d317 |
| EC Number | 68757-95-5 |
| Gmelin Reference | 85359 |
| KEGG | C14111 |
| MeSH | Dichlorophenylpiperazine |
| PubChem CID | 71332 |
| RTECS number | TD6925000 |
| UNII | BE6KM7576D |
| UN number | UN3276 |
| Properties | |
| Chemical formula | C10H12Cl2N2 |
| Molar mass | 248.13 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.3 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.95 |
| Vapor pressure | 3.86E-4 mmHg at 25°C |
| Acidity (pKa) | 8.74 |
| Basicity (pKb) | 5.98 |
| Magnetic susceptibility (χ) | -86.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.627 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 311.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4760 kJ/mol |
| Pharmacology | |
| ATC code | N06AX11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P308+P313, P321, P332+P313, P333+P313, P362+P364, P501 |
| Flash point | 107.4°C |
| Lethal dose or concentration | LD50 oral rat 794 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 1-(2,3-Dichlorophenyl)Piperazine: "250 mg/kg (oral, rat) |
| NIOSH | SNV8750000 |
| PEL (Permissible) | PEL (Permissible) for 1-(2,3-Dichlorophenyl)Piperazine: Not established |
| REL (Recommended) | 10 µg/L |
| Related compounds | |
| Related compounds |
1-(2,4-Dichlorophenyl)piperazine 1-(3-Chlorophenyl)piperazine 1-(2-Chlorophenyl)piperazine 1-(4-Chlorophenyl)piperazine 1-Phenylpiperazine 1-(3,4-Dichlorophenyl)piperazine 1-(2,3-Dimethylphenyl)piperazine |