Chemists started poking around the piperidine ring back in the early 20th century, but the idea to stuff methyl groups onto the piperidin-4-ol backbone picked up momentum in the postwar era, when researchers were chasing stability in oxidation-prone molecules. The burst of synthetic organic chemistry across university labs made it possible to push the limits with bulky amines, especially for those looking to slow down photochemical reactions in new plastics and paints. By the 1960s, advances in N-alkylation and selective methylation let labs tinker with five methyls arranged tightly around the ring, not just improving chemical stability but pushing innovation in field after field. Over time, industrial interest gave rise to scaled-up routes and better characterization tools. Many remember this compound from the days of bustling lab glassware, but today it pops up far from the benches that gave it life.
1,2,2,6,6-Pentamethylpiperidin-4-ol, widely known for its use as a hindered amine, caught my eye in academic and commercial settings. Its crowded structure slows down unwanted reactivity, a critical feature in modern stabilizer technology. A lot of coatings, adhesives, and specialty chemicals rely on its robust profile. The log of applications tells a story: protecting polymers from sun-induced breakdown, enhancing lifetimes of paints exposed to weather, and improving safety profiles for consumer materials. Not every day does a chemical manage to juggle activity and resistance to degradation like this one does.
This compound presents as a solid at room temperature, sporting a waxy or crystalline form depending on purity and processing. With a melting point reported near 62–64°C and a boiling range above 200°C, it withstands reasonable industrial temperatures. Its density sits around 0.96 g/cm³. The standout trait remains the shielded nitrogen atom, courtesy of five methyl groups crowding the scaffold. This arrangement blocks unwanted protonation, limits hydrogen bonding, and gives the compound a stubborn resistance to oxidizing agents and acids. The alcohol group on the fourth ring carbon grants some water solubility, though the hydrophobic bulk dominates. From firsthand experience, handling this molecule calls for respect: its odor is low, but it's persistent—reminders of safety long after a bottle returns to storage.
Manufacturers produce 1,2,2,6,6-pentamethylpiperidin-4-ol to high purity, typically above 98%, with analytical details available on certificates of analysis. Most vendors label containers according to regulatory standards, referencing its unique CAS number and providing hazard information under GHS. Product labels include details like batch number, synthesis date, and storage guidance, reflecting the near-clinical attention to traceability in this segment. Shelf stability lasts, though keeping out moisture and sunlight remains a must. I have seen labeling evolve alongside safety standards, showing how supplier accountability has become a selling point in specialty chemicals.
Building 1,2,2,6,6-pentamethylpiperidin-4-ol starts from piperidine or, more efficiently, from previously substituted piperidines. Synthetic approaches focus on exhaustive methylation, often using methyl iodide or dimethyl sulfate alongside strong bases like sodium hydride or potassium tert-butoxide. Shifting to greener chemistry, some routes now use catalytic processes with less hazardous methyl donors. After methylation, oxidation or hydrolysis techniques introduce the hydroxy group at the 4-position. Careful crystallization and distillation separate out product from byproducts and unreacted material. I’ve seen some labs chase higher yields by switching solvents or pushing for continuous processing on pilot scale—changes that make a world of difference when scaling up.
Thanks to its shielded nature, 1,2,2,6,6-pentamethylpiperidin-4-ol resists most simple acid-base reactions, and the secondary amine rarely forms stable salts. The alcohol moiety can undergo classic transformations: esterification, etherification, and acylation, leading to a suite of derivatives tailored for light stabilization roles. Radical scavenging properties shine in advanced research, and cross-linking via the alcohol allows chemists to attach this fragment to larger macromolecules. Handling in the lab exposes the molecule’s sulfurous side, especially during strong oxidations or reductions, but those who know their way around synthetic routes coax out high yields without too much fuss. Modifications often aim at boosting solubility for specialty applications, a continuous area of practical interest.
On spec sheets, the compound pops up under a handful of names: Pentamethylpiperidinol, 4-hydroxy-1,2,2,6,6-pentamethylpiperidine, and simply PMPOH. Trade names reflect branding decisions by manufacturers working in stabilizer chemistry. Multi-national suppliers provide translated product information, expanding the reach of this niche amine. Staying up to date with synonyms remains important, especially for researchers sifting through patent literature or regulatory guidance—accuracy pays off in both procurement and compliance.
Safety protocols run deep when handling 1,2,2,6,6-pentamethylpiperidin-4-ol. Most workplace guides recommend gloves, protective eyewear, and the use of fume hoods. While acute toxicity registers as low, eye and skin irritation occur if direct contact happens. Stubborn as the molecule is, it doesn’t break down easily, making containment and waste handling priorities, especially in scale-up scenarios. Regulatory bodies such as OSHA and REACH list it with standard precautions, and proper training for handling, storage, and disposal shows up as an industry norm. I have sat in safety briefings where tales of accidental skin contact underline the wisdom in double-gloving and using closed systems—painful lessons that shift company culture for the better.
Industry awards 1,2,2,6,6-pentamethylpiperidin-4-ol a key spot in the formulation of hindered amine light stabilizers (HALS). These agents slow down the harsh effects of UV exposure, a dealbreaker for plastics, automotive coatings, and outdoor paints. Manufacturers blend this additive into synthetic fibers and films looking for long life in sunlight. Beyond plastics, formulators use its derivatives in lubricants, adhesives, sealants, and even cosmetic ingredients seeking to resist oxidation. Few compounds travel so widely between sectors, from construction to sports equipment, a testament to the practical impact of smart molecular design.
Academic and industrial labs dive into new modifications of pentamethylpiperidin-4-ol, hunting for smarter stabilization in next-generation materials. Efforts target better integration into bioplastics and more efficient radical scavenging, responding to strict regulations on microplastics and residue. Research programs lean on analytical tools like NMR, mass spectrometry, and advanced chromatography to tease out impurities and optimize performance. Several innovators chase greener synthesis—cutting down on hazardous methylation reagents or improving catalyst recovery. As someone who has worked on applied polymer chemistry, I’ve seen the pressure to innovate create new cross-sector collaborations, blending academic curiosity with commercial drive for competitive edge.
Toxicologists run regular screening on 1,2,2,6,6-pentamethylpiperidin-4-ol, keeping pace with changing safety standards. Data suggests this molecule avoids acute toxicity in mammalian models, with no major signals for genotoxicity or bioaccumulation under standard tests. Still, long-term studies remain limited, and its persistence in the environment sparks ongoing debate in regulatory circles. Risk assessment departments monitor early signs of irritation or rare allergic reactions in exposed workers, making sure safety data sheets include up-to-date findings. As green chemistry guidelines tighten, researchers monitor breakdown pathways, looking to anticipate and manage any unintended consequences.
The trajectory for 1,2,2,6,6-pentamethylpiperidin-4-ol points to rising demand, especially from sustainable materials sectors. Regulatory focus on environmental fate, worker safety, and product residues pushes companies to develop even more benign analogs—the familiar push-pull between performance and responsibility. Markets expect tighter supply chains, higher purity, and smarter lifecycle management, driven by both consumer awareness and tightening legal frameworks. Research groups lobby for further transparency in toxicity and waste handling, hoping to smooth the integration into greener additives. Those who keep close to the pulse anticipate breakthrough applications in emerging fields like biodegradable polymers, next-gen batteries, and advanced nanocomposites. The push for innovation, blended with real-world caution, keeps this molecule in the crosshairs of scientists and regulators alike.
Most folks outside the science world haven’t heard of 1,2,2,6,6-Pentamethylpiperidin-4-ol. Still, this mouthful of a name describes a chemical that quietly keeps a lot of things working behind the scenes. Chemists count on molecules like this one for a pretty simple reason — nature and industry need ways to fight off the wear and tear caused by oxygen and sunlight. Some of the most annoying problems in products come from breakdown over time, known as oxidation. I’ve had gadgets fail and plastics crumble well before their time, and it all comes down to this invisible process. Products last longer when the right molecules slow those chemical reactions to a crawl.
1,2,2,6,6-Pentamethylpiperidin-4-ol serves as the backbone for a family of substances called hindered amine light stabilizers, or HALS for short. These stabilizers show up all over the plastics industry. If you’ve used a garden chair that sat in the sun and didn’t go brittle, you have molecules like this one to thank. HALS work by trapping free radicals, stopping the chain reactions set off by ultraviolet light. This same reaction helps preserve paints, coatings, and sealants—anything left out in the elements. I remember painting a shed in the backyard and noticing how some brands of paint stood up to sunlight better than others. Digging into the label usually reveals tweaks in the recipe, and some of those tweaks involve stabilizers built with ingredients like 1,2,2,6,6-Pentamethylpiperidin-4-ol.
Studies from both industry and independent labs show that HALS built with piperidine backbones sharply reduce fading, yellowing, and cracking. For example, research published in the Journal of Applied Polymer Science outlines how HALS extend the life of polyolefins, which rank among the world’s most common plastics. The modifications introduced by these stabilizers keep plastics flexible and colors sharp even after thousands of hours exposed to harsh light. According to BASF and other chemical makers, this gives manufacturers a measurable edge — products look new longer, which keeps customers happy and waste out of landfills.
As the world moves toward using fewer resources and making products last, there’s more focus on how additives affect people and the planet. Some stabilizers from decades ago leaked out of plastics and got into the environment. Researchers have kept a close eye on piperidine-based stabilizers like this one, and so far, results show a better safety profile and lower risk of migration compared to older additives. That said, no chemical is completely harmless. Safe handling calls for gloves, goggles, and good ventilation — anyone working hands-on in a factory or lab knows not to skip those steps.
Chemists continue tinkering with piperidine molecules, searching for ways to boost their performance and shrink their environmental footprint. For consumers and businesses, that means more reliable products that cost less to replace. Regulators need to keep pushing for disclosure and safety data, while researchers deliver the detailed studies that show how these chemicals interact with real-world conditions. Upgrading the chemistry behind everyday materials isn’t flashy, but it adds up to a big difference in cost, waste, and quality of life.
Anyone who has spent time working with chemicals knows that a complex name like 1,2,2,6,6-Pentamethylpiperidin-4-ol always deserves a closer look before opening the bottle. This molecule, known in some labs as a hindered amine, finds use mainly in specialty chemical and polymer applications. Folks who have worked in coatings or with light stabilizers might recognize it from the ingredient lists on the back of resin supplies or UV-protectant additives.
Some chemicals burn, corrode, stink you out of the room, or make skin itch on contact. 1,2,2,6,6-Pentamethylpiperidin-4-ol seems subtle. Its physical profile—pale solid, mild scent—doesn’t shout danger. The trouble is, many lab veterans know that the quieter a chemical behaves, the easier it is to forget it can bite back. Its safety data sheet tells the story: this substance causes skin and eye irritation. Some reports list respiratory irritation. That means accidental splashes, dust, or vapors can spark issues. I’ve seen new workers lower their guard around unassuming white powders, but one careless move at the bench can leave you with red, burning skin or a hacking cough. That risk demands respect.
Published studies show this chemical rarely triggers severe reactions in healthy adults under normal conditions. It doesn't rank with acutely toxic solvents or caustic bases, but studies confirm moderate toxicity. Animal experiments highlight irritation but not outright systemic damage at low environmental exposure. Researchers at the National Library of Medicine and European Chemicals Agency classify it as hazardous for eyes and skin, detailing its ability to disrupt cell membranes.
It also sneaks into waterways easily where it lingers longer than other amines, impacting aquatic life. A few mouse studies flag possible liver stress at high doses. That points to the classic “dose makes the poison” situation. Exposure in poorly ventilated rooms, mishandled spills, or large-scale manufacturing can raise risks past the comfort zone of a typical academic or industrial lab.
Most chemists live by a simple creed—treat every chemical like it will get you if you drop your guard. For a substance like this, good gloves, safety glasses, and lab coats should stay in place. I like to keep a small jar with a tight lid for measured samples, so I minimize dust. If there’s a risk of airborne particles, a fume hood makes sense, which I found crucial after a careless shake once left a coworker scraping their eyes for relief.
Anyone with asthma or sensitive skin should pay double attention. Wash any spills quickly. Avoid eating in the lab, no matter how rushed you feel, and keep surfaces clean.
Not every bottle comes with a warning sticker big enough to see across the room. Some workplaces skip training for what feels like niche chemicals, or they rely on outdated safety data sheets. Regulatory agencies update hazard classifications slowly, so it helps to pull data from several databases. I've worked in labs where new hires felt more comfortable around “mild” chemicals until they landed in the nurse’s station. Building habits of careful transfer, storage, and instant cleanup makes a real difference. It helps to check environmental impact reports too—nobody wants to see mismanaged waste harming streams.
The bottom line: treat 1,2,2,6,6-pentamethylpiperidin-4-ol as a moderate hazard, respect basic lab rules, and demand better information from suppliers. That approach keeps hands clean and lungs clear in the long run.
I’ve spent time in labs that handle a bunch of odd chemicals, and I can tell you—most substances have their own quirks. 1,2,2,6,6-Pentamethylpiperidin-4-ol, with its stack of methyl groups sticking out, isn’t just another name in a catalog. This compound, like others with exposed alcohol and heterocycle groups, brings a tendency to react with air and water over time. The walls of every chemical storage room speak with warning stickers, “Keep in a cool, dry place.” That’s not an empty slogan, and it’s not just about following rules.
Warmth speeds up chemical changes. In my own work, I’ve seen what a little heat can do — solids get sticky, bottles crack, fumes fill a cabinet. Pentamethylpiperidin-4-ol should stay below 25°C (77°F). Room temperature suits this, as long as it’s not a greenhouse setting or a sun-drenched windowsill. Even slight temperature bumps in a storage closet kick off slow breakdown, especially in chemicals that absorb moisture or slowly oxidize.
Humidity sneaks in when containers sit open. Anyone who’s handled white powders in muggy weather knows how quickly lumps form, ruining an experiment. Water in the air doesn’t just clump up this compound—it can start a slow series of side reactions that mess with purity. Choosing airtight glass or plastic (preferably amber for light sensitivity) usually saves the hassle. The phrase “tightly closed” isn’t a formality; it guards against not just water but airborne dust that so easily contaminates even small samples.
Light, especially UV rays, encourages unexpected chemical actions. I once watched a clear solution turn yellow after a day in the sun—an easy lesson that some molecules just can’t handle open shelving. This piperidin-4-ol doesn’t shout “photosensitive” as loud as some, still, darkness helps preserve shelf-life. A cupboard with a solid door or a distinctly colored amber bottle goes a long way; keeping the container away from direct beams of sunlight isn’t just for obsessive chemists.
Containers holding larger amounts of any chemical can escalate problems quickly. Spills on a hot afternoon in a poorly ventilated space can lead to headaches—literally and figuratively. Standard safety practice: store with enough space between bottles, label everything, and steer clear of acids, strong oxidizers, or bases. I’ve witnessed more than one near-miss caused by “just tucking an extra sample here” beside an incompatible reagent. Constant organization keeps your bench and your brain uncluttered.
Every workplace with chemical storage keeps a Material Safety Data Sheet on hand—digital access is fine, but printouts near storage areas help in a pinch if the network goes down. These documents, backed by regulatory bodies and reviewed by experienced chemists, offer guidance that has kept more than one lab worker out of trouble. If someone’s wondering about long-term stability or accident cleanup, these sheets serve as a lifeline, not just paper clutter.
The reality of handling 1,2,2,6,6-pentamethylpiperidin-4-ol boils down to simple but crucial habits: cool, dry, darkness, sealed containers, careful labeling, and keeping sources of contamination separate. These steps save money and frustration. They may sound repetitive, but more than once, solid practice has stopped a mistake from turning into an emergency. From my own bench to any chemist setting up shop, treating proper storage like a routine task always pays off.
Anyone who has ever handled organic chemicals can tell you there’s often a risk once the lab work is finished. 1,2,2,6,6-Pentamethylpiperidin-4-ol, often found in specialty synthesis and stabilizer roles, requires proper attention once it’s no longer needed. Its unique ring structure, with five methyl groups, means it doesn’t break down easily in nature. Tossing it down the drain is a quick route to water supply trouble, affecting both wildlife and, eventually, people. EPA studies have shown that compounds in this family persist in waterways, disrupting aquatic life and sometimes triggering toxic byproducts.
For anyone who works in a lab, chemical waste is a part of daily decisions. The Resource Conservation and Recovery Act (RCRA) considers persistent organics and their residues as hazardous until properly neutralized. Fines for improper disposal can wreck both budgets and reputations. More than a few companies have learned the hard way after an inspector dropped in for a surprise visit. I still remember an academic department facing thousands in penalties for after-hours drain dumping, thinking nobody would notice. Lawmakers don’t hand out much leniency in these cases.
I’ve worked in both academic and industry labs, where a solid waste management protocol makes all the difference. The work usually begins with clear labeling. Any bottle or container holding residual 1,2,2,6,6-Pentamethylpiperidin-4-ol earns a red hazardous waste tag. Then it joins a designated collection bin, sometimes with absorbent materials if leaks are a concern. High school chemistry won’t prepare anyone for all the creative ways chemicals can escape from poor seals and crowded benches.
Chemical waste contractors offer pickup services for facilities, and for good reason. Waste-to-energy incineration keeps these compounds out of the ecosystem, breaking down stubborn rings and methyl groups under controlled, high-temperature conditions. Local wastewater plants are not designed to treat synthetic organics with such tenacity. By using commercial incineration, we avoid the slow poison of low-level contamination in soil and streams.
Students and professionals both carry the responsibility for every flask and vial they use. It’s not just about compliance forms or lab inspection day. There’s a certain anxiety that comes with disposing of something that doesn’t belong in our world’s natural cycles. A single lapse—pouring unneeded chemicals down the drain with hopes of anonymity—can haunt water systems for generations. Garbage isn’t invisible once it leaves the benchtop; someone else, sometimes far downstream, pays the price.
Responsible chemical disposal needs resources and training. Universities and companies with strong safety programs teach newcomers exactly how to segregate, label, and store hazardous organic waste. I’ve seen beginner mistakes turn into learning moments: a senior researcher calmly walking someone through the process, explaining what’s at stake. A few posters and regular reminders help shape habits. Training sessions should include real stories, not just theoretical risks, so new workers take the guidance seriously. Creating that culture takes ongoing vigilance—safety officers, department chairs, and every student or staff member have a role. For most, once you see the impact of careless habits, careful disposal becomes second nature.
Choosing the safest disposal route means protecting more than just the workplace. It signals respect for health—our own, our coworkers’, our neighborhoods’. When the journey of a chemical ends responsibly, we keep the cycle clean, letting the lab’s discoveries remain a force for good, not for unintended harm. Real stewardship comes from treating every milliliter, every leftover batch, as more than just waste—it’s a chance to do right by others who share the world’s water, soil, and air.
Every molecule tells a story—1,2,2,6,6-Pentamethylpiperidin-4-ol has roots in the world of organic chemistry that are simple to appreciate once brought down to earth. Here’s how it shapes up. The backbone is a piperidine ring, which scientists often describe as a six-membered loop with one nitrogen tucked in among the carbons. Attached to this ring are five methyl groups—bulky little carbon clusters capped with hydrogen—set on the first, second (twice), and sixth (twice) positions. At the fourth position, an -OH group bonds with the ring, creating the “ol” in its name—a classic sign of an alcohol functional group.
Looking at the way atoms arrange themselves in this molecule, something stands out beyond the superficial formula. The piperidine ring is shaped to bring stability, a core trait for compounds that need to stick around through rough treatment—bright light, strong winds, even the acids and bases that show up in chemistry labs and industry settings. Those methyl groups do more than puff up the structure; they block unwanted attacks from other chemicals, making the molecule tougher to break down. It’s like packing a box with extra padding to keep things safe inside.
Getting too abstract gives chemistry a bad reputation. In my experience, this molecule matters because it acts as a steric shield—a bulked-up bouncer resisting nasty reactions that chew up lesser compounds. For chemists, this makes 1,2,2,6,6-Pentamethylpiperidin-4-ol a reliable building block in industrial processes. Plastic stabilizers and light protectant additives rely on its persistence. It doesn’t just sit on a shelf; it stands guard in real-world products, increasing the lifespan of plastics and coatings under sun and heat.
Safety matters as much in the factory as in the lab. Truth is, bulky molecules like this one behave very differently compared to smaller, more mobile organic compounds. Its structure keeps it from breaking down easily—sometimes a blessing, but also a potential issue if it finds its way into the air or water outside controlled settings. For example, persistent chemicals can accumulate, and close tracking becomes necessary. It reminds me of the way persistent pesticides showed up decades later in unlikely places; chemical stability often outlasts best intentions.
Fact-based choices should lead how we use compounds like 1,2,2,6,6-Pentamethylpiperidin-4-ol. Green chemistry practices suggest ways to manage risk—like recycling waste and setting strict disposal rules. One approach worth attention is molecular design that makes breakdown just as dependable in the environment as in the lab, offering the benefits of chemical resistance while limiting the mess downstream.
Complex names can push people away from questions, but unraveling the structure of 1,2,2,6,6-Pentamethylpiperidin-4-ol gives a clear view into why it matters. Decisions about how these molecules enter daily life deserve respect for both science and the environment—backed by industry vigilance and continuous research to balance performance and stewardship.
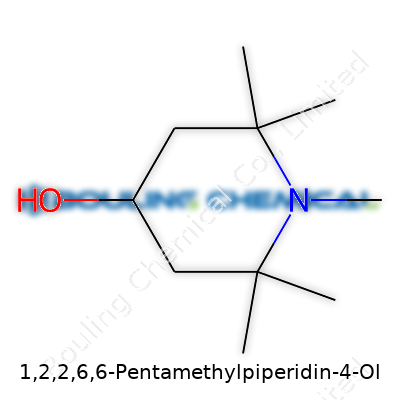
| Names | |
| Preferred IUPAC name | 4-Hydroxy-1,2,2,6,6-pentamethylpiperidine |
| Other names |
4-Hydroxy-1,2,2,6,6-pentamethylpiperidine 4-Hydroxy-TEMPO Triol HO-TEMPO TEMPOL |
| Pronunciation | /ˌwʌnˌtuːˌtuːˌsɪksˌsɪksˌpɛntəˈmiːθəlpaɪˈpɛrɪdɪnˈfɔːrɒl/ |
| Identifiers | |
| CAS Number | 1136-75-4 |
| Beilstein Reference | 1221932 |
| ChEBI | CHEBI:44470 |
| ChEMBL | CHEMBL572330 |
| ChemSpider | 21360826 |
| DrugBank | DB08245 |
| ECHA InfoCard | ECHA InfoCard: 100.086.593 |
| EC Number | 208-761-5 |
| Gmelin Reference | 88242 |
| KEGG | C06424 |
| MeSH | D018044 |
| PubChem CID | 72186 |
| RTECS number | SE1037500 |
| UNII | 81ON1J8P5S |
| UN number | UN No. 2810 |
| CompTox Dashboard (EPA) | DTXSID3039242 |
| Properties | |
| Chemical formula | C10H21NO |
| Molar mass | 157.28 g/mol |
| Appearance | White solid |
| Odor | Aromatic |
| Density | 0.896 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.97 |
| Vapor pressure | 0.15 mmHg (20°C) |
| Acidity (pKa) | 19.78 |
| Basicity (pKb) | 4.89 |
| Magnetic susceptibility (χ) | -7.91E-6 cm³/mol |
| Refractive index (nD) | 1.483 |
| Viscosity | 67 mmHg (80°C) |
| Dipole moment | 1.89 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 132.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -380.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7248.6 kJ/mol |
| Pharmacology | |
| ATC code | D02AE01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | >110 °C |
| Autoignition temperature | 225 °C (437 °F; 498 K) |
| Lethal dose or concentration | LD50 (oral, rat): 3020 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 5000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Piperidine 2,2,6,6-Tetramethylpiperidine 4-Hydroxypiperidine N-Methylpiperidine N,N-Dimethylpiperidine TEMPO (2,2,6,6-Tetramethylpiperidin-1-oxyl) 1,2,2,6,6-Pentamethylpiperidine |