Chemists have always searched for flexible reagents that could simplify peptide synthesis, fine-tune protection strategies, and drive new transformations in organic chemistry. By the late 20th century, the need for safer alternatives to the classic but risky thiocarbonyl sources like thiophosgene had grown urgent. Researchers began to turn their attention to bis-imidazoles, already valued for creating convenient leaving groups and activating carboxyls with minimal side reactions. In that spirit, 1,1'-Thiocarbonylbis(imidazole) (TCBI) emerged as a stable, solid reagent that could introduce thiocarbonyl groups without the unpredictable hazards of older, gaseous compounds. Its adoption rapidly expanded from specialized laboratories to wider use in academic and industrial settings due to its robust performance, storage ease, and improved safety profile.
1,1'-Thiocarbonylbis(imidazole) brings thiocarbonyl functionality in an accessible, manageable form. Chemists recognize TCBI’s bright yellow crystalline appearance as a sign of purity and potency. The molecule efficiently delivers thiocarbonyl units for the formation of thioesters, thiocarbamates, and other sulfur-containing derivatives. Academic labs, peptide synthesis ventures, and specialty chemical manufacturers all find a solid partner in TCBI, whether handling milligram reactions or planning upscaled, kilogram-level syntheses. Its reliability across conditions means confidence in outcomes and more productive days at the bench.
TCBI appears as a vividly yellow crystalline powder, sparing users the mess and volatility of liquid alternatives. Its melting point ranges from 128°C to 133°C. Solubility in common organic solvents—dichloromethane, DMSO, and acetonitrile—makes it adaptable during setup. Unlike moisture-sensitive or toxic predecessors, TCBI endures standard handling with reasonable care. Its stability at room temperature allows storage out of cold rooms but it demands protection from prolonged humidity or direct sunlight. Chemical analysis confirms its purity via sharp NMR signals and robust IR absorptions characteristic of thiocarbonyl imidazoles, granting peace of mind before each use.
Suppliers usually provide TCBI at 95% or higher purity, listing CAS number 6160-65-2 on labels alongside standard hazard warnings. Batch-specific analysis sheets cover melting point ranges, residual solvent content, and key spectra. Labels often include GHS pictograms for irritancy, given the mild risks associated with thiocarbonyl reactivity and imidazole exposure. Exact storage suggestions, typical packaging options (sealed amber bottles, desiccation for larger lots), and expiration guidance ensure safe, effective inventory management in both research and industry settings.
Preparation usually starts with imidazole reacting directly with thiophosgene or, more modernly, with hexamethyldisilathiane as a sulfur transfer agent. In practical synthesis, thiophosgene gets dissolved in an inert solvent like dichloromethane, cooled, and then slowly combined with an excess of imidazole. The product separates by filtration and undergoes recrystallization from ethanol or ethyl acetate for purification. Yields routinely exceed 80%. USB analysts and academic process chemists favor this route for its efficiency, predictability, and the manageable byproduct profile. Waste streams can be neutralized or captured through established work-up steps, setting a reasonable safety baseline compared to traditional thiocarbonyl routes.
TCBI shines as a mild, controllable source of the thiocarbonyl group. Adding it to alcohols or phenols in the presence of a base forms thiocarbonates or thionocarbonates in high yield, opening the door to carbon-sulfur bond construction in medicinal chemistry and polymer science. Amines react to build thiocarbamate intermediates without harsh temperature swings. Peptide chemists value TCBI in mild thioesterification, enabling native chemical ligation for peptide and protein assembly. Its compatibility with a diversity of nucleophiles and protection groups helps when planning multi-step syntheses that call for sequence or orthogonality.
Besides its IUPAC alias, 1,1'-Thiocarbonylbis(imidazole) pops up as TCBI, Imidazole-1-carbothioic acid imidazolide, and Imidazole thionocarbonyl. Product catalogues may reference it as TCI T0582, Sigma-Aldrich 233533, or ACROS 170810050, depending on the supplier and batch. This handy network of synonyms ensures that whatever the source, procurement officers and bench chemists can locate and track TCBI across documentation and inventory.
TCBI, though far safer than volatile thiophosgene or carbon disulfide, commands respect. It can irritate skin and eyes, especially in fine dust form, so lab workers suit up with gloves, eye protection, and use chemical hoods during weighing and dissolution. Respiratory masks stay close by for larger preparations. Material safety data sheets advise keeping TCBI sealed from prolonged moisture, as slow hydrolysis may liberate small amounts of imidazole and hydrogen sulfide. Disposal follows standard protocol for sulfur-containing organic compounds: neutralization if dissolved or incineration through licensed waste facilities. Training focuses on spillage response, accidental contact, and safe long-term storage.
Peptide, carbohydrate, and nucleoside synthesis groups rely on TCBI’s mild yet effective chemistry for building sulfur-bridged intermediates. Industrial manufacturers use it while producing bioactive thioesters and specialty fine chemicals for crop protection or pharmaceutical pipelines. In research settings, TCBI streamlines protocols for forming challenging thionated structures, which appear in dyes, sensors, and protease inhibitors. Material scientists leverage TCBI in the quest to design new conductive or optical polymers bearing strong, stable C=S bonds. Its user-friendly nature encourages adoption beyond niche laboratory circles to more mainstream chemical processes.
Recent research dives deeper into green chemistry alternatives for TCBI’s preparation, aiming to sidestep thiophosgene with enzymatic or safer sulfuration methods. Analytical chemists experiment with TCBI in solid-phase procedures that promise automated, rapid construction of libraries containing thiocarbonyl components. Synthesis groups see value in TCBI as part of novel protection and activation strategies—especially where traditional thiolating agents slow or complicate multistep syntheses. Ongoing collaborations between academia and industry push to broaden its reactivity scope, expand its use in cross-coupling applications, and streamline post-reaction purification.
Comprehensive toxicity data highlight TCBI’s advantages compared to earlier thiocarbonyl reagents. Its solid, crystalline form and lower vapor pressure reduce inhalation risk. Standard in vivo studies point to mild to moderate toxicity, driven by imidazole’s metabolic byproducts rather than acute, immediate poisoning. Contact studies show reversible skin and ocular irritation with direct exposure, placing TCBI firmly in the “handle with regular PPE” class rather than emergency hazard. Environmental agencies encourage minimum-waste procedures, as sulfurous breakdown products can harm aquatic systems if released in bulk. Long-term occupational health reviews currently show no major chronic risk, but prudent monitoring of exposure remains best practice.
Interest in safer, greener thiocarbonyl transfer continues to drive innovation with TCBI as a focal point. Industrial trends lean toward replacing gaseous thiophosgene and chlorinated reagents wherever possible. Researchers explore TCBI analogues that deliver even tighter selectivity or faster reaction profiles, envisioning tools for making polythioesters, cyclic thionolactones, and custom small molecules unavailable through older techniques. Conversation within the chemical community shows hope for process intensification—smaller waste streams, less solvent usage, and straightforward recycling—all areas where TCBI or its improved cousins could anchor future manufacturing. Continued investment in toxicity research, alternative routes, and scale-up solutions promises to keep TCBI relevant for decades to come, appealing to both classrooms and global production lines.
Chemists like me often reach for certain reagents when a specific transformation is needed in the laboratory. 1,1'-Thiocarbonylbis(imidazole), or TCBI, has carved out a particular spot on the shelves of those who work in organic and medicinal chemistry. Its main job revolves around converting alcohols and amines into the corresponding thiocarbonyl compounds, especially in the production of thioesters, thioamides, and thiocarbamates. The value in this transformation comes from the way sulfur atoms can be used to fine-tune the biological properties of molecules, something pharmaceutical research leans on heavily.
Anyone who’s tried to turn an alcohol into a thioester knows that it doesn’t always go smoothly. Older methods call for hazardous reagents like thiophosgene, which come with strict handling rules and safety nightmares. TCBI offers a way to skip those headaches. In my own work, using TCBI meant I could carry out key steps under milder conditions – no toxic gas, no bulky fume hood, and much less risk of nasty byproducts that have to be scrubbed out later.
It's not just about convenience. Researchers at places like Pfizer and Novartis keep betting on TCBI because the compounds that result are critical building blocks for drugs against cancer, viruses, and inflammatory disease. Modifying a molecule to include a sulfur atom can make it bind more tightly to an enzyme, dodge breakdown in the liver, or sneak past cell membranes. You need reliable, selective reagents to do this work — and TCBI checks those boxes.
Handling chemicals should never be an exercise in risk-taking. Thiocarbonylbis(imidazole) simplifies usually tricky reactions. Lab accidents cut down dramatically when you swap out the older, more dangerous reagents for something that’s available as a powder, stable at room temperature, and much easier to portion out. Undergrad students can use it in teaching labs with standard gloves and goggles, which means the next generation of chemists can actually learn from experience, not just from books.
There’s always room for improvement in the cost department, since specialty reagents don’t come cheap, especially at scale. Teams have worked on making TCBI with less waste and from more abundant starting materials to address this. Making cheaper, greener, and safer sulfur reagents ticks a lot of boxes for both industry and academia. More sustainable options will drive broader adoption, especially in places where budgets run tight.
The main use for TCBI now sits at the intersection of chemical innovation and drug discovery. Each new medicine starts with a pile of candidates, and small changes to a side chain or backbone — like swapping out an oxygen for a sulfur with TCBI — can turn a dud into a blockbuster. It’s this “what if” mentality that keeps the work fresh and productive. I see TCBI becoming even more important as chemists push for safer, more reliable tools to deliver the next generation of treatments.
Better availability and awareness will only help researchers further explore sulfur chemistry. This means more options for patients, fewer production hiccups, and a safer environment for scientists. TCBI reminds us that the right tools don’t just make our work easier — they shape the future of medicine.
Every time someone runs a synthesis in the lab, there’s that expectation of getting clean results. In the rush to push through steps, it’s easy to underestimate the risks with chemicals like 1,1'-Thiocarbonylbis(Imidazole). Years hunched over fume hoods taught me that familiarity brings comfort, but it also opens the door for mistakes. This reagent packs a punch—powerful for activating carboxylic acids, unforgiving if you ignore safety.
1,1'-Thiocarbonylbis(Imidazole) fits in the family of reactive acylating agents. Handling can result in itchy skin, rashes, or burning eyes if it gets out of line. Inhaling dust or vapors? Welcome to lung and throat irritation. Chronic exposure doesn’t just tweak your comfort—it can potentially cause serious health slides. I watched new lab members replay the same errors, and that’s why training should be non-negotiable, not a formality.
Gloves aren’t just a nice suggestion. This compound can zip through nitrile in no time, so switching gloves often becomes second nature. Two layers, latex over nitrile, serve better if you’re in for a long session. Finding the right goggles stopped the eye-watering that comes with poorly ventilated benches. Lab coats need to stay buttoned and cover as much skin as possible.
Ventilation isn’t some checkbox to tick on a safety audit. One summer, our old lab’s extraction fan broke. We learned quickly how stifling rooms filled with traces of volatile chemicals become. Sashes down on fume hoods and working at the back of the workspace make a difference you feel in your breathing at the end of the day.
Dry, cool, and away from direct sunlight keeps 1,1'-Thiocarbonylbis(Imidazole) stable. Seals on containers matter more than people think—moisture sneaking in degrades the reagent and spikes reactivity. If you skip proper closure, it cakes up and might even pressure-pop when you open it later. Keep incompatible substances far away. Acids and bases, especially, don’t mix well with it in any scope.
I once saw a new tech go pale after knocking over a bottle. Calm reactions matter more than shame. Scoop up spills using absorbent pads and plenty of ventilation. Never use water to clean up, as it reacts fast and releases heat. Bag contaminated material and label it for hazardous waste. Disposal down the drain turns a small mistake into a major environmental problem.
Regular safety refreshers catch attention before habits slip. Having emergency showers and eyewash stations checked and ready means nobody hesitates to use them. One time, a colleague delayed flushing their eyes, and the burn lingered for days. Notifying emergency contacts quickly lowers risk when accidents happen. Every lab benefits when people speak up about unsafe setups instead of waiting for an inspection.
Technology creates smarter airflow monitors and alerts for chemical leaks now. Labs adopting these tools stay safer, especially during long or overnight reactions. New PPE materials resist permeation better than older versions, cutting down risk. Safety walks and collective ownership build trust and raise safety from a rule to a habit.
Experience in the trenches teaches that respect for 1,1'-Thiocarbonylbis(Imidazole) begins long before the first weigh-in and lingers after cleanup. Taking these precautions protects not just the chemist, but everyone sharing a space or handling the waste downstream.
1,1'-Thiocarbonylbis(Imidazole) never shows up in daily life for most people, but in a lab, it turns into an essential reagent for introducing thiocarbonyl groups. What surprises many is how easily people gloss over safety basics, especially for something with reactivity on par with this compound. My first time handling this powder, a mentor looked me in the eye and said, “This isn’t sugar. Store it like you want a paycheck next week.” That stuck with me.
The literature describes 1,1'-Thiocarbonylbis(Imidazole) as moisture-sensitive. Exposing it to air, even for a short while, pushes it to react or degrade. Extra caution is more than a recommendation. Any sign of humidity in the storage area cuts shelf life and can create hazards. I recall a time a carelessly sealed vial developed clumps after just a week, turning a smooth workflow into a risky affair.
This is why it goes straight into a tightly-sealed bottle, preferably with a screw cap lined with PTFE. Keepers of chemistry stockrooms often stash these bottles inside a desiccator, sometimes doubling up with silica gel. It might feel like an extra step, but corroded caps and strange odors prove those steps matter.
Heat encourages unwanted chemical changes. Room temperature feels safe, but heating vents in storage closets and summer afternoons add subtle yet steady warmth that nudges compounds to break down faster. At my old workplace, dedicated chemical refrigerators became the go-to solution, keeping most moisture-sensitive or reactive powders cool and stable, typically around 2–8°C.
1,1'-Thiocarbonylbis(Imidazole) plays by those same rules. As a bonus, cooler temperatures reduce the speed of air and moisture absorption for anything accidentally left out. Always label the bottle with the date opened, and stick to storing it away from light, which may degrade some organic compounds over time.
A clear label with the full name, hazard warnings, and date of receipt does more than satisfy inventory managers. In shared workspaces, confusion over which bottle holds which solid can spark real trouble. I’ve seen a near-miss when someone mistook one white powder for another—labels and color-coded tape dodged a disaster.
The U.S. Occupational Safety and Health Administration (OSHA) lists several requirements for handling sensitive or hazardous powders, including airtight containers, limited exposure, and secondary containment. Following this guidance, well-ventilated storage spaces, ideally away from busy foot traffic, create another layer of safety. The Globally Harmonized System (GHS) assigns pictograms that remind users about risk: health hazard, corrosive, or flammable, depending on the chemical.
Teaching new lab members how to store compounds like 1,1'-Thiocarbonylbis(Imidazole) forms a crucial step in onboarding. Watching a careful technician build good habits serves as a living reminder—write down any observations, replace desiccant regularly, and note odd smells or discoloration.
If a lab team keeps these practices in mind—airtight bottles, dry environments, cool temperatures, strong labeling, and a culture of double-checking—chemical storage moves from an afterthought to part of the workflow. With compounds like 1,1'-Thiocarbonylbis(Imidazole), it only takes one shortcut for things to go sideways. Reputation, safety, and good results start with respect for the bottle before anything hits a reaction flask.
1,1'-Thiocarbonylbis(imidazole) doesn’t show up on most people’s radar. Chemists, especially those dealing with synthetic pathways, see this compound more often than anyone else. Its chemical formula, C7H6N4S, tells us quite a bit before we even pull out the calculator. It gives insight into how the molecule pieces together, why it’s chosen for certain reactions, and what its weight means for anyone working in the lab.
Looking at the atomic weights in the periodic table, carbon stands at about 12.01, hydrogen at 1.01, nitrogen at 14.01, and sulfur lands at 32.07. Summing up, two imidazole rings, a sulfur, and a linker group combine their weights. Here’s the breakdown: carbon (7 atoms) contributes 84.07, hydrogen (6 atoms) gives 6.06, nitrogen (4 atoms) with 56.04, and sulfur adds 32.07. These add up to a molecular weight of roughly 178.24 grams per mole. This value lines up with professional chemical catalogs and widely-used scientific references.
Precision in chemistry starts with knowing exactly how much of a compound you’re using. Weighing out 1,1'-Thiocarbonylbis(imidazole) for a reaction means that every decimal point in the molecular weight makes a difference. Small errors here can throw off stoichiometry, especially since labs rarely work with giant excesses of materials. Over time, I’ve found that quality work in research comes back to these basics—accurate weighing, correct calculations, and understanding the outcome of the reaction.
This compound turns up in peptide chemistry, but its uses aren’t confined there. Any protocol involving thiocarbonyl transfer or certain functional group introductions might reach for it. Using the correct molecular weight doesn’t just keep a chemist honest, it maintains reproducibility, supports peer-reviewed work, and allows projects to scale from tiny vials to industrial vats. I’ve seen start-up labs save time and money just by double-checking such fundamental details.
A mistake with the molecular weight drags down the whole synthesis. Product yields drop, impurities creep in, or downstream reactions don’t work as planned. In the pharmaceutical world, mistakes with calculations like this can cost millions or, in rare cases, risk patient safety. In academic labs, students experience frustration and failed thesis projects simply because someone misread a number in a reagent book.
Online resources update fast, but not everyone learns to double-check with primary sources. I always recommend cross-checking with reputable suppliers like Sigma-Aldrich or Tokyo Chemical Industry. Their published molecular weights come vetted, updated, and supported by their global customer base. If discrepancies pop up, don’t just trust the first hit on a search engine—take a minute to pull data from a chemical structure or run the numbers by hand. Students in my classes often build confidence by learning these skills the hard way.
Understanding the molecular weight of compounds like 1,1'-Thiocarbonylbis(imidazole) forms a foundation for laboratory work that’s reliable and transparent. By sticking to high-quality references, using calculators or software to check work, and training eyes to spot outliers, every chemist can improve their daily practice. These details set apart labs where projects run smoothly and papers get accepted, from those where avoidable mistakes slow both research and production.
1,1'-Thiocarbonylbis(Imidazole) doesn't show up on most people's radar, but those who’ve stepped into a chemistry lab, even once, know every detail counts. Its name alone hints at the kind of stuff chemists wrangle daily—compounds with long, tongue-twisting labels doing jobs behind the scenes. This particular compound lives in the world of organic synthesis, helping turn simple starting materials into useful molecules by activating carboxylic acids, essentially kicking chemical reactions into gear.
Drop 1,1'-Thiocarbonylbis(Imidazole) into a glass of water, and you’re not going to see it melt away like sugar or salt. Scientific data backs this up: most reputable databases and chemical suppliers report this substance as practically insoluble in water. The chemistry isn’t hard to understand. Its core structure—a thiocarbonyl group linked between two imidazole rings—simply doesn’t play well with water molecules. With very little ability to form hydrogen bonds and a molecular structure leaning toward nonpolar, the compound prefers to stick with other organics instead of spreading in a polar solution like water.
Years back, working with organic synthesis reminded me how water—or lack of it—forces a hands-on approach. Water solubility decides not just where you can use a compound, but how cleanly you can separate out products. If a reagent stays in the organic layer, you worry less about tricky purifications and side-reactions from water. In the lab, 1,1'-Thiocarbonylbis(Imidazole) won this point—it fits right in with organic solvents. Straightforward separation, fewer unexpected byproducts, and often-times higher yields curl out of the test tube.
From a safety angle, low water solubility also eases environmental pressure. Spills don’t dissolve and soak right into water systems. Cleanup feels less urgent, but waste management doesn’t get off easy. The problem shifts—the compound doesn’t just vanish; it might stick around longer in the environment or in organic wastes, so proper disposal remains crucial.
Scientists can’t force solvents to behave. Instead, they plan syntheses to match the quirks of the reagents. 1,1'-Thiocarbonylbis(Imidazole) shines in solvents like dichloromethane and acetonitrile. Protocols steer away from water, capitalizing on its strengths. If someone needs to use this in a mixed solvent setup, they rely on emulsifiers or clever extraction tricks, though that often adds time and cost. Some chemists keep hunting for water-friendly analogues, on the chance they’ll open greener options—but not every chemistry problem bends so easily to green chemistry’s wishes.
For academic researchers, knowing these limitations at the start saves time and money. Mistakes teach quickly: I’ve watched a stack of precious starting material disappear into a failed reaction because I misunderstood the solvent compatibility. Choosing the wrong solvent can ruin a day's work or an expensive experiment. Clear documentation on what dissolves and what doesn’t protects both the science and the scientist.
In practical terms, those looking to work with 1,1'-Thiocarbonylbis(Imidazole) should skip water altogether. Stock it, store it dry, and plan to dissolve it only in classic organic solvents. Good lab notes, up-to-date safety sheets, and an eye on disposal stop it from becoming a problem compound. Rushing into a synthesis without enough checking risks not just failed experiments, but bigger headaches with cleanup, worker safety, and downstream waste treatment.
As new synthetic techniques push toward more sustainable chemistry, solubility will keep playing its silent but starring role. For every breakthrough process, the question never fades: does this dissolve where you need it—or does it stay stubbornly in the wrong place? The answer shapes everything that follows.
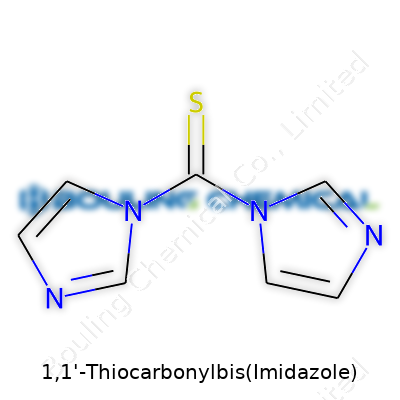
| Names | |
| Preferred IUPAC name | Imidazole-1-carbothioimidate |
| Other names |
TCDI Carbonothioic diimidazole Imidazole-1-carbothioic acid anhydride Thiocarbonyldiimidazole |
| Pronunciation | /ˌwʌn wʌn θaɪ.oʊˌkɑːr.bə.nɪl.bɪs.ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | [3546-41-6] |
| Beilstein Reference | 3408735 |
| ChEBI | CHEBI:51509 |
| ChEMBL | CHEMBL22237 |
| ChemSpider | 91421 |
| DrugBank | DB08242 |
| ECHA InfoCard | 100.024.093 |
| EC Number | 613-449-4 |
| Gmelin Reference | 78412 |
| KEGG | C14332 |
| MeSH | D017978 |
| PubChem CID | 169870 |
| RTECS number | KH9375000 |
| UNII | FG1R83H365 |
| UN number | 3261 |
| CompTox Dashboard (EPA) | DTXSID4040987 |
| Properties | |
| Chemical formula | C7H6N4S |
| Molar mass | 265.34 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.31 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.19 |
| Vapor pressure | <0.01 mmHg (20°C) |
| Acidity (pKa) | 9.45 |
| Basicity (pKb) | 8.15 |
| Magnetic susceptibility (χ) | -54.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.625 |
| Viscosity | 2.143 mPa·s (20°C) |
| Dipole moment | 6.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -71.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -831.7 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: "Wear protective gloves/protective clothing/eye protection/face protection. Avoid breathing dust/fume/gas/mist/vapors/spray. Wash thoroughly after handling. IF ON SKIN: Wash with plenty of water. |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | > 221°C |
| LD50 (median dose) | LD50 (median dose): 960 mg/kg (rat, oral) |
| NIOSH | SY8225000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1,1'-Thiocarbonylbis(Imidazole): Not established |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Carbonyldiimidazole Thiophosgene Carbonothioic dibromide |