Chemists looking for ways to make better peptide bonds set the scene for the invention of 1,1'-Carbonylbis-1H-imidazole, better known as carbonyldiimidazole or CDI. Researchers first reported it in the 1950s, using it to activate carboxylic acids for amide bond formation. Early publications in the 1960s detailed its efficiency and selectivity, letting labs skip messy acid chlorides. Word spread quickly among organic chemists, and over the years CDI evolved from an obscure lab chemical into a bread-and-butter reagent for peptide synthesis and beyond. It showed clear promise for easy, high-yield couplings without toxic byproducts, kicking off decades of creative research.
CDI comes as a white or off-white crystalline powder, often packed in airtight glass or HDPE bottles. Its CAS number is 530-62-1. Many labs—not only academic but also in the biopharma and polymer fields—keep it on their shelves for carbamate, urea, and ester synthesis. Industry-wise, CDI goes far beyond peptide chemistry, finding a role in process development for pharmaceuticals, polymer cross-linking, and even surface functionalization for analytical tools. Its shelf life depends on strict dryness, since CDI reacts impatiently with any traces of water.
1,1'-Carbonyldiimidazole melts at roughly 117-119°C, and dissolves well in aprotic solvents like dimethylformamide, acetonitrile, and dichloromethane. It tolerates many organic solvents, though ethanol or water spoil its performance. With a molecular weight of 162.16 g/mol, its chemical formula reads C7H6N4O. In direct sunlight or in the presence of water vapor, CDI may decompose. The molecule’s central carbonyl group, flanked by two imidazole rings, forms the perfect set-up for activating acids or alcohols. Its reactivity, combined with a relatively stable shelf life (if stored away from humidity), makes it a mainstay in custom synthesis.
Suppliers typically sell CDI at 98% purity or higher, and label bottles with warnings about moisture sensitivity. Certificates of analysis show melting point, assay, and residual solvents. GMP-grade CDI may feature additional testing for heavy metals and residual solvents. Package sizes span from 25 grams for routine lab work up to kilogram quantities for industrial reactors. Labels carry the proper hazard pictograms, indicating harmful and irritant potential.
Industrial manufacturers make CDI by treating phosgene with imidazole, but safer lab-scale routes swap phosgene for triphosgene, letting chemists avoid handling gas. The process uses dry solvents and a non-aqueous workup. After reaction, vacuum or rotary evaporation removes excess solvent, and careful recrystallization ensures a high-purity product. Chemists value the reproducibility and straightforward purification, which helped CDI make the leap from niche to mainstream.
CDI’s best-known role is activating carboxylic acids for coupling with amines, forming amides under mild, near-neutral conditions. Reaction with acids generates an N-acylimidazole intermediate, which then reacts with the amine. CDI also helps convert alcohols and amines into carbamates, ureas, and other derivatives. Polymer scientists, for example, use CDI to introduce active esters on biopolymers, enabling cross-linking or attachment of peptides. Analytical chemistry labs modify surfaces with CDI to attach ligands to chromatography resins. Modifications of the parent CDI molecule—like N-substituted imidazoles—aim to tweak reactivity or alter solubility.
CDI goes by several synonyms, which can confuse less experienced chemists. Common names include carbonyldiimidazole, 1,1'-Carbonylbis(imidazole), and imidazole carbamate. In catalogs, some brands refer to it as CDI, others as carbonimidazole, though the CDI acronym (for carbonyldiimidazole) prevails in research papers and patents.
Working with CDI needs a respect for its moisture reactivity—any exposure to water or humid air wastes reagent and gives unwanted byproducts. CDI causes skin and eye irritation, so chemists keep gloves and goggles on at all times. Standard operating procedures urge use in a fume hood to avoid inhaling fine particulates. Storage requires silica gel or a desiccator cabinet. Disposal involves neutralization with alcohol and water under controlled conditions, as CDI hydrolyzes to carbon dioxide and imidazole.
Synthetic organic chemistry claims the bulk of CDI applications, using it to make peptide bonds, amide linkages, and urea groups. Medicinal chemists rely on CDI to build libraries of peptides and peptidomimetics without harsh reagents. Bioconjugation workflows make use of its ability to form stable linkers between proteins and dyes or drugs. Material scientists apply CDI to functionalize surfaces, produce hydrogels, and tune polymer properties for tissue engineering. QC labs deploy CDI for efficient compound derivatization in HPLC and GCMS analysis. People see its flexibility and reliability as drivers of its popularity in academic and industrial projects alike.
Recent efforts in R&D try to further expand CDI’s reactivity and sustainability. Green chemistry teams look for alternatives to the phosgene process, aiming for safer starting materials and less waste. Researchers work on water-compatible CDI derivatives for bio-conjugation in living systems. Publications highlight efforts to automate CDI-based coupling in flow reactors, enabling high-throughput synthesis for drug discovery. Collaboration between academic labs and industry propels CDI-related methodologies that handle increasingly complex natural products and biologics.
Toxicologists examined CDI exposure, noting its potential for skin, eye, and respiratory irritation. Chronic toxicity remains low compared to many coupling reagents, but direct contact or inhalation of dust definitely poses risks. CDI is not classified as mutagenic or carcinogenic according to major chemical safety agencies, but safe handling and effective ventilation remain necessary. By-products such as imidazole pose limited risk, as they break down rapidly in the environment. Research looks at eco-friendlier alternatives, mainly for large-scale use where waste minimization and safer handling gain extra weight due to high throughput.
The outlook for CDI and related reagents keeps gaining complexity thanks to new frontiers in drug discovery, materials science, and bioengineering. Teams working on “green” CDI aim for even milder reaction conditions, better compatibility with biotechnologies, and less hazardous production methods. Next-gen biotech companies experiment with CDI to functionalize proteins, enzymes, and DNA on preparative scales. Process chemistry now drives automation projects, seeking to use CDI in integrated, continuous systems for efficient pharmaceutical synthesis. As applications expand into tissue engineering, diagnostics, and advanced polymers, demand persists for improvements in safety, sustainability, and precision. CDI’s role as a linchpin in synthetic chemistry remains strong—by supporting further research, funding, and smarter regulation, its relevance will only grow.
Ask any lab worker what chemicals make life easier, and you’ll notice 1,1'-Carbonylbis-1H-imidazole (CDI) gets a nod every time. CDI shows up most in labs where researchers need to piece molecules together, especially for hooks between carboxylic acids and amines. In other words, CDI isn’t some exotic compound gathering dust. It hits the bench to help form peptides, various esters, and complex amides — all staples in drug development and organic chemistry.
Peptide coupling usually brings to mind tricky conditions: harsh reagents, side reactions, and a treasure chest of byproducts. CDI steps in as the gentle fixer. By converting a carboxylic acid into a much more reactive intermediate (the imidazolide), CDI lets the amine partner waltz right in and form an amide with hardly any waste to clean up. No need to wrestle with corrosive chlorinating agents or make big safety trade-offs.
Working with CDI in the lab gives peace of mind. The reagent stores easily, handles well even in drafty teaching labs, and skips the stench or volatility that chase students out for a break. For peptide makers — from university researchers to drug companies — that spells more hours at the bench and fewer in cleanup gear. On top of that, the reactions stay mild, so sensitive molecules don’t break down. Many green chemistry advocates have written up comparisons showing that CDI produces mostly CO2 and imidazole as byproducts — a relief compared to toxic alternatives like carbodiimides.
Beyond just making medicines, CDI’s value keeps growing. Biotech companies reach for it when building diagnostic tools or labeling proteins. Materials science researchers work with polymers and coatings that need clean amide links — CDI slots in perfectly. Whenever synthesis calls for reacting carboxylic acids and alcohols (making esters), CDI still delivers without flooding the flask with strong acids.
Even with its clear perks, CDI creates hurdles if the water creeps in — it breaks down quickly to carbon dioxide and imidazole, leaving chemists racing to dry glassware thoroughly. Not all acids and amines play nice, either. Bulky or weakly nucleophilic partners still call for heat or extra time. And in scaling up, handling imidazole waste sometimes eats into the project’s environmental credentials.
Still, my own experience running gram-scale amide couplings with CDI has shown me how it dodges nasty byproducts. We get higher yields, especially with precious or delicate molecules, and rarely see racemization — a big issue for peptide chemists chasing single-enantiomer drugs.
Improved drying techniques save reactions from moisture. Streamlined protocols using automated synthesisers now curb human error with CDI, making scale-ups safer and smoother. Smart waste recycling — isolating imidazole for reuse — offers a sustainability boost for teams producing on an industrial scale.
As labs evolve, tweaks to CDI’s structure could cut down side reactions even more. Research groups keep mapping new activation modes that let chemists bypass some of the old speed bumps. CDI’s primary use — helping amines and acids form tight, reliable bonds — won’t fade soon.
1,1'-Carbonylbis-1H-imidazole is a chemical professional researchers—especially those in organic chemistry—see quite often. Used for peptide synthesis and as a coupling agent, it can make chemical reactions smoother. Yet, this old workhorse can cause headaches if someone ignores basic safety or shrugs off proper storage guidelines.
My own experience has taught me that this compound acts like a sponge for moisture. If left out, or if someone stores it in a loosely capped bottle, cloudiness or clumping starts fast. This change hints at hydrolysis—water breaking down the molecule—rendering it useless for sensitive reactions. Keeping it in a tightly sealed container makes all the difference. Many chemists stash it in glass, high-density polyethylene, or amber bottles with a secure seal, not because of fancy protocols, but because they’ve seen too many grams wasted by a novice mistake.
A dry environment holds equal value. Desiccators or cabinets with silica gel prolong shelf life and keep costs under control. On more than one occasion, I watched a student reach for an old sample, only to find a ruined white powder—money wasted simply because they skipped the dry box.
Keeping carbonylimidazole at room temperature works, yet that means something different to each lab. In tropical climates or during summer, benches can hit 28°C or even higher, placing the compound’s stability at risk. I’ve learned to keep it in cool, shaded cabinets, never near hotplates or windows. For bulk storage, the best place remains a refrigerator or cool room, preferably at 2–8°C, but not frozen. Too cold, and condensation sneaks in after opening the bottle—another chance for hydrolysis.
Mixing strong acids, bases, or humid reagents near or with carbonylimidazole spells trouble. I recall an incident in which someone opened the bottle near concentrated hydrochloric acid—within a week, the material showed signs of decomposition. Segregate it from incompatible chemicals by shelf or even by lab bench side. People who value their time and safety usually group carbonylimidazole with other dry, stable organic materials.
Direct contact with carbonylimidazole warrants respect. It can cause severe eye and respiratory irritation. I always put on safety goggles, gloves, and work with it in a fume hood. Small spills respond well to sweeping up and washing with large amounts of water, but only if handled immediately. Nobody in the lab enjoys sticky gloves or stinging skin, so even on a lazy Friday, covering up is worth the few extra seconds.
Labels help, but real risk reduction comes from practical habits. I store measuring tools separately for compounds like carbonylimidazole—cross-contamination can sabotage a week’s work of experiments. I clean scales and work surfaces as soon as I finish. This looks like overkill to outsiders, yet avoiding accidental exposure and ensuring clean results pays off, time after time.
Clean storage and careful handling are not “red tape.” They reflect lessons learned from ruined experiments and minor lab accidents. Lab safety isn’t just about ticking boxes. Looking after reagents like 1,1'-carbonylbis-1H-imidazole, caring for your colleagues, and protecting your experiments—which could save months of work—mean relying on routine, straightforward, no-excuse discipline. That’s how you keep the science, and the people, going strong.
Every chemist faces the puzzle of dissolving stubborn reagents. 1,1'-Carbonylbis-1H-imidazole, often called CDI, ranks high on the problem-solver’s list in peptide synthesis and coupling reactions. Over years in the lab, experience quickly teaches which solvents coax CDI to dissolve and which leave a frustrating, suspended solid.
Take CDI and try to stir it into water—the result will look unchanged even after hours on a stir plate. CDI stays practically insoluble in water. Even common alcohols like methanol, ethanol, and isopropanol give lackluster results. You’ll find only a trace will dissolve. Chemistry students learn early: CDI sticks to organics for a reason. Its hydrophobic nature explains why you barely see any CDI go into solution with water.
Things change with solvents like dichloromethane (DCM), tetrahydrofuran (THF), and ethyl acetate. Here, CDI shows moderate solubility. In the lab, a gram or two melts away into the liquid with a decent stir. DCM and THF offer enough polarity to loosen up CDI’s crystalline structure. Rotovaps run non-stop with these choices, especially during peptide workups. DMF, the notorious all-purpose solvent, brings out CDI’s best behavior. Scientists see it dissolve freely in DMF. You want high concentration and fast reactions? Reach for DMF—its polar, aprotic character lines up with CDI’s chemistry. DMSO matches DMF with CDI, and gets used just as often for trickier, less-reactive substrates.
Solubility impacts more than just a chemist’s day. At the research bench, a poorly chosen solvent drags out reaction times, leads to partial conversions, or leaves you scraping product off filter paper. In industry, CDI’s compatibility with DMF or DMSO streamlines scale-up. Companies avoid solvents like water or alcohols at large scale not out of preference, but out of real-world necessity—low solubility equals high waste and inefficiency. Poor solubility often spikes costs with extra agitation, longer reaction times, or larger solvent volumes.
Every solvent brings trade-offs. DMF and DMSO dissolve CDI beautifully, but both have raised eyebrows for health and environmental reasons. On a busy synthesis floor, handling these solvents requires gloves, hoods, and careful disposal. Chemists keep one eye on OSHA regulations and another on evolving green chemistry trends. Ethyl acetate or acetonitrile show moderate performance with CDI and offer friendlier toxicity profiles, so chemists try to swap these in for smaller scale syntheses.
If safety and sustainability keep you awake at night, new generations of solvents may help. Dimethyl carbonate and 2-methyltetrahydrofuran promise similar performance with fewer regulatory headaches. Sometimes a blend of solvents tips the solubility scale just enough, striking a balance between CDI’s needs and a chemist’s peace of mind. Small changes in solvent choice echo into waste streams, worker safety, and regulatory burdens. That makes every liter of solvent matter.
What chemists really need are clear, reliable solubility tables from hands-on lab data. Vendor brochures provide ballpark figures, but the best lessons come from keeping good lab notes and sharing real outcomes, not just values lifted from handbooks. The story of CDI’s solubility is one told by both molecules and the people wrestling them into solution, always looking to do good science with fewer headaches and greater care for the world around us.
Working with 1,1'-Carbonylbis-1H-Imidazole (CDI) brings certain risks that chemists shouldn’t brush off. CDI enables peptide coupling and other useful organic reactions, but those perks come with baggage. Touching or inhaling this compound can spark some real trouble. Skin gets irritated. Eyes feel that sting. The powdery solid throws off dust, making it easy for tiny particles to float into the air and land where they shouldn’t—lungs, eyes, even your coffee if you aren’t careful.
Beyond the obvious irritation, CDI breaks down in water and humidity, unleashing imidazole fumes and carbon dioxide. Simple chemistry, big mess. The fumes sneak into the room, especially if ventilation fails or someone leaves the container open. Breathing in the product or its byproducts brings a burning sensation in the nose and throat; it also hurts the lungs. Left unchecked, accidental spills or dust clouds in a tight workspace could lead to headaches, coughing, or worse.
It only takes one lesson to realize eye goggles aren’t optional. I once saw a colleague skip them—only to scramble for the eyewash a minute later. Not all outcomes end with quick recoveries. The biggest risk stews in the receptor chemistry labs, where containers get opened, and powders bounce around from scoopulas or spatulas. Without diligence, contamination spreads to doorknobs, gloves, and work jackets. Even tiny traces carry enough punch to spark irritation, as proven by rash outbreaks in labs that ignored glove changes.
Research shows CDI reacts with water to make carbon dioxide and imidazole, and the reaction speeds up at higher temperatures. So I always store it in airtight containers, tucked inside a desiccator. Labeling makes a difference, too. I’ve found that people treat “danger – powder may irritate” more seriously than “store dry.” Staff who switch gloves every time they finish using CDI cut down accidental contact. Experience shows that once people tape down warning notices or add colored tape to lids, the whole team keeps their guard up.
Working in a fume hood stands as a core practice. Fume hoods vent away CN or imidazole rich dust that might slip past a face mask. Ordinary surgical masks miss those particles. Full face shields, disposable coveralls, and double-gloving go a long way, especially during weighing or transfer. Even simple habits—wiping benches with damp cloths after working, keeping hands away from faces, avoiding snacks in the lab—lower the odds of accidental exposure.
Chemsafety databases list CDI as a moderate to severe irritant. In practice, that means even veteran chemists run risk unless they treat every use like their first. New staff should get walk-throughs. Our lab built a culture of reviewing accidents together and changing policies, not just scolding mistakes. By updating checklists and swapping stories on best and worst moments, we keep everyone plugged in. Regular drills, sharing PPE types that work best, and a policy of never working with CDI alone all reinforce a safe environment.
CDI helps move science forward, but only by treating its hazards with respect does real progress happen. Every ounce of caution pays off—far more than patching up accidents after the fact.
In the lab, plenty of us have come across 1,1'-Carbonylbis-1H-Imidazole—also known simply as CDI. It's a trusty reagent for peptide synthesis, coupling reactions, and making activated esters. Most chemists who have handled it know that stability isn’t just a backdrop to success; it makes or breaks an experiment. Keeping your CDI fresh ensures dependable results, and nobody wants to gamble with purity after months in storage.
Years of practice show there’s one thing CDI simply doesn’t get along with: water. Even a hint of humidity in the air starts CDI down a path of hydrolysis, splitting the molecule to imidazole and carbon dioxide. That means you lose reactivity, and your synthetic plans start slipping out of your control. Exposing CDI to air over and over again is a surefire way to cut its shelf life painfully short. Even in a tightly capped amber bottle, an open-and-close routine chips away at its quality.
Fresh CDI, straight from the manufacturer, typically carries a shelf life of two years if stored under nitrogen in an airtight container and kept cool—usually at or below 25°C. That’s the number you’ll see printed on safety sheets from reputable suppliers. Chemically speaking, it’s not because the molecule decays with time, but because contamination slows over on a warm shelf or in open air. Based on published research and my day-to-day work, folks who store CDI below freezing—say, in a -20°C freezer—notice little decline in performance even after three years. In contrast, leaving a bottle in a humid stockroom can cut working time down to less than six months.
You don’t need fancy analytics to spot lousy CDI. It usually goes from a crisp white powder to a yellowish, clumpy mess as it breaks down. You’ll know something’s off if your reactions stall, or you need more reagent than usual to get comparable yields. High-performance labs run a melting point or thin-layer chromatography check, but if in doubt, trust your eyes and your gut. High moisture or a musty smell signals trouble.
No one wants to shell out for new chemicals every quarter. If your work relies on CDI, toss small packets of desiccant in storage jars—silica gel gobbles up any loose moisture. For folks who demand peak performance, store your supply in a glove box or under a dry nitrogen blanket. If breaking a seal for every use can't be helped, divide your purchase into smaller vials to avoid exposing the whole lot. Educating colleagues about the risks does more than any written protocol. Experience says the cost of a little prevention beats the pain of failed syntheses and rerunning reactions.
CDI can stay usable far beyond the printed expiration if treated right, though playing chicken with expiration dates courts disaster. Regular checks and common sense habits—a cool, dry, airtight world—outlast formal guidelines. It’s not about fancy storage—it’s about respect for the fleeting nature of so many useful reagents. Quality reagents lead to quality research, and nobody builds reliable science on crumbling foundations.
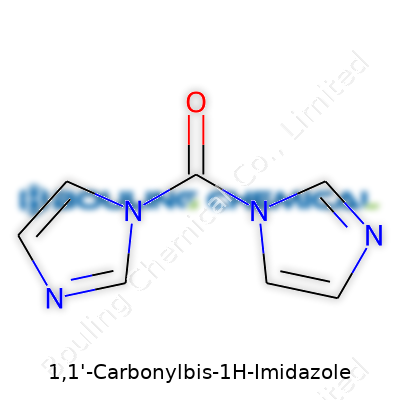
| Names | |
| Preferred IUPAC name | 1,1'-[Carbonylbis(azanediyl)]di(1H-imidazole) |
| Other names |
Carbonyldiimidazole CDI Imidazole-1-carboxylic acid, anhydride 1,1’-Carbonyldiimidazole 1,1’-Carbonyldimidazole N,N′-Carbonyldiimidazole |
| Pronunciation | /ˈkɑːr.bə.nɪl.baɪs.wʌn.eɪtʃ.ɪˈmɪd.əˌzoʊl/ |
| Identifiers | |
| CAS Number | 530-62-1 |
| Beilstein Reference | 64724 |
| ChEBI | CHEBI:39073 |
| ChEMBL | CHEMBL14240 |
| ChemSpider | 21522 |
| DrugBank | DB03653 |
| ECHA InfoCard | ECHA InfoCard: 100.023.776 |
| EC Number | 206-819-0 |
| Gmelin Reference | 10422 |
| KEGG | C07285 |
| MeSH | D000077261 |
| PubChem CID | 3477 |
| RTECS number | KW2975000 |
| UNII | N07T50I5TY |
| UN number | 3261 |
| CompTox Dashboard (EPA) | DTXSID80893803 |
| Properties | |
| Chemical formula | C7H6N4O |
| Molar mass | 222.19 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.44 g/cm³ |
| Solubility in water | slightly soluble |
| log P | -0.23 |
| Vapor pressure | <0.00001 hPa (20 °C) |
| Acidity (pKa) | 11.4 |
| Basicity (pKb) | 5.32 |
| Magnetic susceptibility (χ) | -61.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.554 |
| Viscosity | 1.621 mPa.s (20 °C) |
| Dipole moment | 5.4746 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 329.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -110 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1073.7 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V03AX26 |
| Hazards | |
| Main hazards | Causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | Flash point: 182°C |
| Lethal dose or concentration | LD50 oral rat 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 940 mg/kg |
| NIOSH | FF1925000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g |
| Related compounds | |
| Related compounds |
Imidazole 2-Methylimidazole Histidine Benzimidazole |